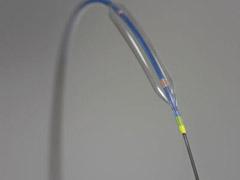
May 25, 2010 – A balloon catheter technology with enhanced trackability and a redesigned tip for greater flexibility was recently cleared by the U.S. Food and Drug Administration (FDA) and received European CE mark. The noncompliant (NC) Quantum Apex percutaneous transluminal coronary angioplasty (PTCA) dilatation balloon catheter is being launched in European this week and the United States in June.
The NC Quantum Apex Catheter is a high-performance, post-dilatation balloon catheter developed specifically to address physicians’ needs in optimizing coronary stent deployment. Boston Scientific said it represents the next generation of its balloon catheter technology. It is available in a wide array of balloon diameters from 2 to 5 mm, with balloon lengths ranging from 6 to 30 mm. The monorail catheter platform will be available worldwide and both the monorail and over-the-wire (OTW) catheter platforms will be available in the United States.
The NC Quantum Apex Catheter performed very well, offering a noticeably lower profile, excellent trackability and effective post-dilatation,” said Jean Fajadet, M.D., director of the interventional cardiology, Clinique Pasteur, Toulouse, France. He was the first physician to treat patients with the new balloon catheter. “It features an innovative design and impressive performance that should benefit interventional cardiologists and their patients.”
Bruce Brodie, M.D., interventional cardiologist, Moses Cone Heart and Vascular Center, and chairman, LeBauer Cardiovascular Research Foundation, Greensboro, N.C., served as principal investigator of the POSTIT study. He was one of the first physicians in the United States to use the catheter. “Results from the POSTIT clinical study showed that more than 70 percent of coronary stents are not optimally deployed by a stent delivery balloon alone,” said Brodie. “The use of IVUS (intravascular ultrasound) with an adjunctive post-dilatation balloon makes it twice as likely that a stent will be optimally deployed. The NC Quantum Apex Catheter is a great addition to the available post-dilatation balloons, making it easier for physicians to achieve optimal stent deployment.”
For more information: www.bostonscientific.com


 June 13, 2024
June 13, 2024 









