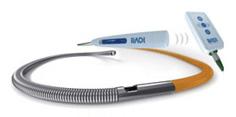
The wireless PressureWire Aeris FFR measurement system.
March 17, 2010 – A new agreement with Siemens Medical Solutions expands access to the wireless PressureWire Aeris fractional flow reserve (FFR) measurement technology. St. Jude Medical also announced several updates to its PressureWire platform this week at ACC 2010.
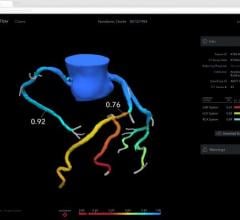
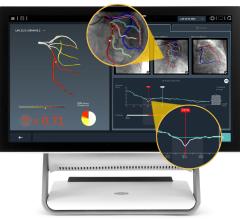
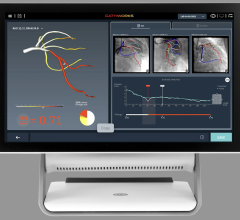
The new marketing agreement integrates the wireless FFR system with Siemen’s AXIOM Sensis XP cath lab hemodynamic recording system to immediately display, measure and save FFR data. This documents the severity of coronary lesions, together with other procedural data and angiographic imagery. The wireless technology of the PressureWire Aeris also eliminates cables crossing the sterile field.
Because of this new agreement with Siemens, and existing compatibility with other recording systems including the GE Mac-Lab Hemodynamic Recording System, Mennen Horizon XVu and the McKesson Horizon Cardiology Hemo solution, the PressureWire Aeris technology can be used in the majority of cardiac cath labs for wireless integrated FFR measurement utilizing existing hardware.
The new version of the PressureWire Certus FFR wire was also introduced at ACC. It includes modifications to design and functionality that will provide physicians with more controlled handling and versatility. It is the only guide wire on the market to offer in one wire the combined measurement of pressure and temperature, which enables calculations of FFR, coronary flow reserve (CFR) and an index of microcirculatory resistance (IMR).
The PressureWire Certus was the only FFR measurement system used in the landmark FAME (Fractional Flow Reserve (FFR) vs. Angiography in Multivessel Evaluation) trial, which found both superior clinical outcomes and reduced healthcare costs in patients whose treatment was based on FFR.
For more information: sjm.com


 February 03, 2026
February 03, 2026