October 21, 2009 – W. L. Gore and Associates today announced the first human implants of the next generation Conformable GORE TAG Thoracic Endoprosthesis for the treatment of thoracic aortic aneurysms (TAAs).
The first implants were performed by Joshua Rovin, M.D. and Eugene Murphy, M.D., at Bayfront Medical Center and William McMillan, M.D. and Scott Schultz, M.D., at North Memorial Medical Center. The devices were used to treat patients with a TAA, which is an enlargement that develops in weakened areas in the thoracic aorta.
Gore received approval of an investigational device exemption (IDE) from the FDA to investigate the use of the next generation Conformable GORE TAG Thoracic Endoprosthesis in TAAs. William Jordan, MD, from the University of Alabama, Birmingham, will serve as the national principle investigator in the Conformable GORE TAG Device in Thoracic Aortic Aneurysm Trial (Gore TAG 08-03). “This study will evaluate device performance across the wide portfolio of sizes available with the next generation Conformable GORE TAG Device,” Dr. Jordan said. The next generation device portfolio includes device diameters ranging from 21–45 mm, as well as tapered devices, and the study will investigate the treatment of patients with aortic diameters of 16–42 mm.
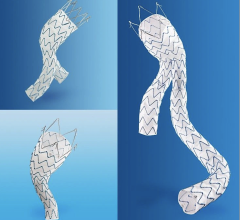
The commercially available GORE TAG Thoracic Endoprosthesis provides a minimally invasive option for safely and effectively treating patients with aneurysms of the descending thoracic aorta (DTA). It is comprised of an ePTFE graft with an outer self-expanding nitinol support structure to combine both device flexibility and material durability. It received pre-market approval from the FDA in 2005.
In addition to TAAs, the next generation Conformable GORE TAG Device has been approved to investigate endovascular repair of other etiologies including traumatic aortic transection and aortic dissection. Design enhancements include a modified stent frame, repositioned gold bands, and optimized graft material.
For more information: www.goremedical.com

 April 26, 2023
April 26, 2023