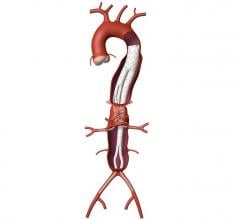
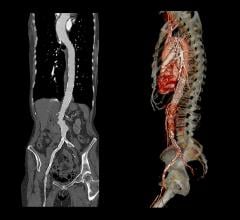
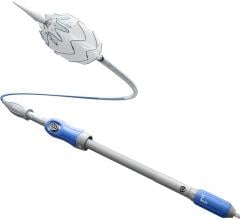
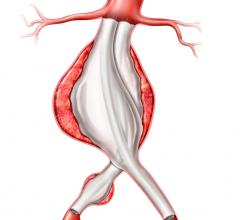
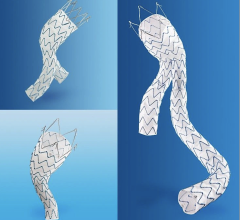
The Valiant Stent Graft
October 12, 2009 – Medtronic Inc. recent received CE mark and began its international launch of the Captivia Delivery System for the Valiant Thoracic Stent Graft, a minimally invasive treatment for aneurysms and other lesions of the thoracic aorta.
The Captivia Delivery System features tip capture for enhanced control of the Valiant Thoracic Stent Graft during deployment and a hydrophilic coating applied to the graft cover to facilitate iliac access and delivery through patients’ vasculature. Medtronic said the new system enables physicians to treat a wide range of anatomies with a highly conformable stent graft, with accuracy and ease of delivery to achieve optimal clinical results. Both the Valiant Thoracic Stent Graft and Captivia Delivery System are investigational in the United States, where their use is limited to clinical trials approved by the U.S. Food and Drug Administration.
Professor Giovanni Torsello, chief of vascular surgery at St. Franziskus-Hospital in Muenster, Germany, performed the first Valiant Thoracic Stent Graft implantation with the Captivia Delivery System. “The Captivia Delivery System’s tip capture feature provides excellent control of the stent graft during deployment, which is critically important when treating lesions in the thoracic aorta,” Dr. Torsello said. “Its hydrophilic coating has also made a significant improvement in stent graft delivery. These added features will provide greater confidence in physicians’ ability to treat these very challenging cases.”
Indicated for the treatment of a variety of thoracic aortic lesions, the Valiant Thoracic Stent Graft has emerged as the minimally invasive “system of choice” for thoracic endovascular aortic repair (TEVAR) outside the United States. In four years of clinical experience, more than 15,000 patients worldwide have received the Valiant Thoracic Stent Graft.
For more information: www.medtronic.com

 April 26, 2023
April 26, 2023