September 16, 2009 – MemorialCare Heart and Vascular Institute at Long Beach Memorial Medical Center (LBMMC) in Long Beach, Calif., said it implanted a new, heart attack detection device in two heart attack survivors yesterday.
With the danger of a second heart attack occurring in the first year for 35 percent of female survivors and 20 percent of male survivors, the device is designed to monitor and analyze data about a patient’s heart, reducing the time it takes to get to the emergency room. Long Beach Memorial is one of only 16 U.S. hospitals currently participating in the pilot study for the AngelMed Guardian device. The two patients, David Leland and Bruce Fisher, are the first west of the Mississippi to undergo the procedure.
“A second heart attack within the first year of survival is very common and unfortunately, most patients don’t go to the emergency room until three hours after symptoms start,” said John Messenger, M.D. “Alerting patients that they need immediate medical attention before it’s too late could profoundly change heart attack survival rates. Long Beach Memorial continuously looks for ways to apply innovative technologies that are focused on prevention, and the Guardian device has the potential to save many lives during a time critical emergency.”
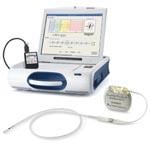
The cardiac monitor and alert system is comprised of an internal implantable device about the size of a standard pacemaker with a lead into the heart, a pager device, and a programmer that aids physicians in evaluating heart signals. Patients wear the pager at all times and are alerted by a combination of vibration, beeps, and a flashing light to notify them to see their doctor or go immediately to the ER.
“After my first heart attack, I knew that I was extremely susceptible to another episode,” said David Leland. “I chose to participate in this trial program because I trust the physicians at Long Beach’s MemorialCare Heart and Vascular Institute, and truly believe that this device will give me an advantage if symptoms reoccur.”
The objective of the ALERTS Pivotal Study is to provide an assessment of the safety and effectiveness of the AngelMed Guardian System. The company began the pivotal trial in October 2008 to seek FDA approval.
For more information: www.angel-med.com, www.memorialcare.org


 January 05, 2026
January 05, 2026 









