August 11, 2009 – Physicians in the United Kingdom yesterday completed patient implants using the first CE mark approved drug-eluting stent designed specifically to treat severe peripheral artery disease (PAD) blockages in the challenging and largest artery in the leg.
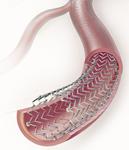
Cook’s polymer-free Zilver PTX Drug-Eluting Peripheral Stent is specifically designed and approved to treat PAD affecting the main blood vessel in the thigh, the superficial femoral artery (SFA). It is a self-expanding stent made of nitinol, a space-age, shape memory metal that offers unique mechanical advantages for a stent in the SFA, the company said.
By eliminating the need for a polymer agent to hold the drug to the stent body, Cook has created a medical breakthrough that solves two key problems. First, it allows targeted delivery of a drug (paclitaxel) to reduce restenosis of arteries opened using balloon angioplasty. Second, by eliminating the need for a polymer, which was left behind on the body of earlier drug-eluting stents after the drug dissolved into the surrounding tissues, Zilver PTX avoids the potential patient risks posed by leaving a permanent foreign, plastic substance in the body. In addition, the Zilver stent was proven during its clinical trial to be the most durable peripheral stent available, suggesting even greater patient safety, according to the clinical trial data, Cook Medical said.
The CE mark follows the world's largest clinical trial for a peripheral stent, led by Michael Dake, M.D., professor in the department of cardiothoracic surgery at Stanford University Medical School and medical director of the cath/angio laboratories at Stanford University Medical Center, Palo Alto, Calif. The data collected in the Zilver PTX registry involved 791 patients from Europe, Russia, Canada, and Korea and demonstrated highly positive results. Only 8 percent of patients with de novo lesions needed a reintervention to reopen the artery in the first 12 months – a rate significantly surpassing existing treatments for PAD in the SFA, such as balloon angioplasty and bare metal stents, the company said.
Also, specific patient groups that are often very hard to treat, such as diabetics and patients with in-stent restenosis, were shown in the trial to benefit from the Zilver PTX. As the trial data indicate, the superior results achieved in the first year have been largely maintained throughout 24 months. In comparisons with other trials published, the Zilver PTX stent showed a reduction in reintervention of between 50 percent and 75 percent, Cook Medical said.
“The awarding of the CE mark is set to herald a revolution in the treatment of peripheral arterial disease,” said Dr. Dake. “This global study proves that the Zilver PTX has the integrity, safety, and durability needed to successfully address many of the well-known limitations of current treatments for the management of PAD.”
Following more than 1,200 patients treated worldwide during its clinical evaluation and CE mark approval in July 2009, the first commercial implantations of the Zilver PTX stent were conducted yesterday in a coordinated effort by physicians in the United Kingdom, Germany, France, Holland, Belgium, Sweden, Switzerland and Spain. In the United States, the Zilver PTX drug-eluting stent is an investigational device not available for sale.
For more information: www.cookmedical.com


 July 02, 2024
July 02, 2024 









