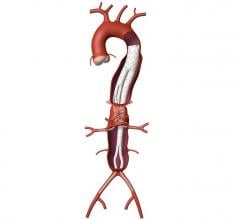
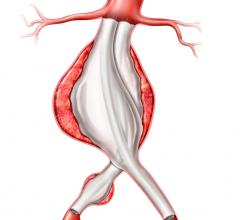
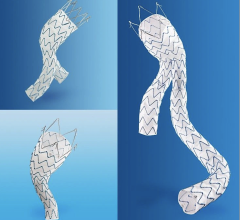
July 27, 2009 – Terumo Cardiovascular Systems said Friday it initiated a phase II clinical trial for the Anaconda AAA (abdominal aortic aneurysm) stent graft system in the U.S.
The Anaconda system is manufactured by Scotland-based Terumo subsidiary Vascutek Ltd. The first U.S. implant was performed on June 8 at Arizona Heart Hospital by the principal investigator, Julio Rodriguez-Lopez, M.D.
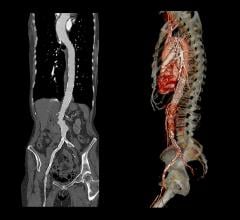
The objective of the Vascutek Anaconda Stent Graft System Phase II IDE Study (G030036) is to assess the safety and effectiveness of the Anaconda in patients presenting with AAA when compared to historical open surgical repair. The FDA gave Terumo CVS approval to enroll 180 patients at 20 sites, some of which will be in Canada. The primary endpoint for the study is the successful treatment of the aneurysm 12 months after the implantation. Patients in the study will be followed for a total of five years.
The Anaconda system is commercially available outside the U.S. where it has been implanted in nearly 4,000 patients. It received CE Mark in Europe in April 2005.
For more information: www.terumo-cvs.com

 April 26, 2023
April 26, 2023