March 11, 2009 - Results from the PROTECT I trial evaluating the feasibility and effectiveness of the Abiomed Inc. Impella 2.5 circulatory assist device in high-risk percutaneous coronary intervention (PCI) procedures were published in the February issue of the Journal of the American College of Cardiology (JACC).
The study, "A Prospective Feasibility Trial Investigating the Use of the Impella 2.5 System in Patients Undergoing High-Risk Percutaneous Coronary Intervention (The PROTECT I Trial)" concludes, "The Impella 2.5 system is safe, easy to use, and provides excellent hemodynamic support during high-risk PCI [percutaneous coronary intervention]."
The PROTECT I trial enrolled 20 patients undergoing high-risk PCI at seven centers between July 2006 and April 2007. Eligible patients had left ventricular ejection fraction (EF) of less than 35 percent and were required to undergo PCI on either an unprotected left main coronary artery or the last patent coronary conduit.
"The Impella 2.5 is poised to change the standard of care in our efforts to combat heart disease and its devastating after-effects," said Igor F. Palacios, M.D, director of interventional cardiology, Massachusetts General Hospital, Boston, associate professor of medicine at Harvard University Medical School and participating cardiologist in the Protect I trial. "The PROTECT I trial enrolled a very sick patient population and demonstrated that the device works and validated its impressive safety profile, showing no valve, blood or vascular damage, no instances of stroke and a low adverse event rate."
Hospitals enrolling patients included Academic Medical Center, Brigham and Women's Hospital, Cedars Sinai, Massachusetts General Hospital, Scripps Clinic, Texas Heart Institute and William Beaumont Hospital.
William W. O'Neill, M.D., professor and executive dean for clinical affairs at the Leonard M. Miller School of Medicine, University of Miami was the principal Investigator for the study.
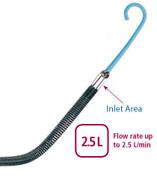
The Impella 2.5 received 510(k) clearance from the FDA in June 2008 for partial circulatory support for periods up to six hours. It is inserted percutaneously in the catheterization lab via the femoral artery into the left ventricle. Up to 2.5 liters of blood per minute is delivered by the pump from the left ventricle into the ascending aorta, providing the heart with active support in critical situations. Now approved in more than 40 countries, including in Europe under the CE Mark, Impella 2.5 has been used to treat over 1,700 patients worldwide and has been the subject of more than 50 peer-reviewed publications, the company said.
" Abiomed is also currently conducting two U.S. pivotal studies comparing the Impella 2.5 to the IABP (Protect II for high-risk percutaneous coronary intervention, or PCI; and Recover II for acute myocardial infarction).
For more information: http://interventions.onlinejacc.org/cgi/content/full/2/2/91, www.abiomed.com.


 January 05, 2026
January 05, 2026 









