July 24, 2008 - The FDA has granted clearance for GE Healthcare’s newest positron emission tomography/computed tomography (PET/CT) scanner, which is part of GE’s Discovery family of scanners designed to enable earlier detection and monitoring of disease with advanced molecular imaging technology in both hardware and software.
The new system will be optimized for use in oncology, which represents more than 90 percent of clinical PET/CT exams. The system continues using the highest-sensitivity crystals in the industry, along with GE’s exclusive VUE Point HD, High Definition imaging to help clinicians advance towards the goal of Motion Free PET/CT imaging.
“The newest PET/CT scanners are an important step toward further reducing the effects of motion and improving the physician’s ability to help patients through better clinical images,” a company spokesperson said.
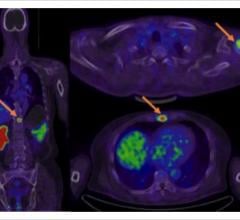
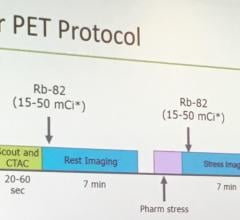
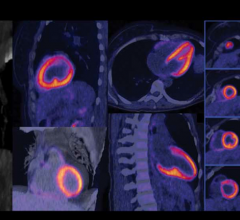
Motion generated by the patient’s respiration can blur small lesions affecting resolution and quantitative accuracy. VUE Point HD, combined with other GE-exclusive motion-management techniques help improve lesion detectability, treatment planning and quantitative accuracy, improved contrast-to-noise of moving lesions up to 60 percent versus static acquisition for overall better clinical confidence.
The company will ship the first new scanners to customers in the fourth quarter of this year.
For more information: www.gehealthcare.com


 June 23, 2025
June 23, 2025