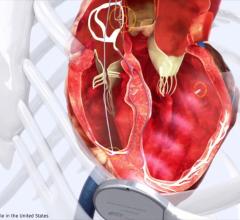
April 14, 2008 - Boston Scientific Corp. reported that the Japanese Ministry of Health, Labor and Welfare (MHLW) approved ACUITY Steerable left ventricular lead for use with cardiac resynchronization therapy (CRT) devices, which treat heart failure.
The Company, which also received reimbursement approval for the lead from the National Health Insurance System, plans to launch the product immediately in Japan.
The ACUITY Steerable left ventricular lead features a deflectable tip for successful delivery in simple, compound and complex anatomies, which includes placement of the lead in difficult-to-access branch vessels on the left side of the heart. A lead is an insulated wire that carries the heart signal to the implanted device and delivers energy from the device to the heart. In most cases, leads are passed into the heart through veins.
The ACUITY Steerable lead also provides physicians with four configurations for stimulating the left side of the heart, which Boston Scientific refers to as Electronic Repositioning. This ability allows physicians to change the stimulation site non-invasively after implant, which helps avoid the need for an additional surgical procedure. Boston Scientific is the only company that provides physicians with four left ventricular pacing configurations.
For more information: www.bostonscientific.com


 July 21, 2025
July 21, 2025