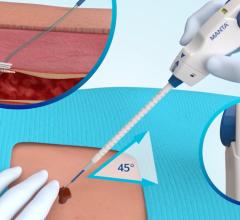
August 29, 2007 - St. Jude Medical Inc. announced that it has received regulatory approval from the Ministry of Health, Labor and Welfare (MHLW), in addition to reimbursement approval, for the Angio-Seal STS Plus Vascular Closure device, strengthening the companies position in the international vascular closure device market.
Angio-Seal STS Plus is designed to provide physicians with an improved method of sealing catheterization sites, allowing patients increased comfort and offering hospitals improved efficiencies. Approved for both percutaneous peripheral and cardiac interventional catheterization procedures, the device enables physicians to quickly seal femoral artery punctures made during those procedures.
According to the manufacturer, with the addition of a self-tightening suture to the design, the Angio-Seal STS Plus allows vascular closure to be completed entirely in the catheterization lab, saving labor and reducing the length of time required to complete the procedure.
For more information: www.sjm.com


 October 07, 2025
October 07, 2025