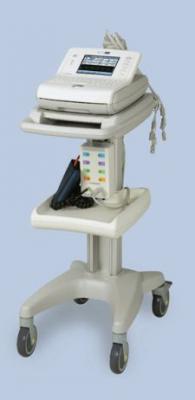
CardioDynamics received market FDA clearance for its new BioZ Impedance Cardiography (ICG) clinical parameters and electronic medical record (EMR) interface capability for its BioZ Dx System.
The new parameters include total arterial compliance which allows the assessment of peripheral artery elasticity, and Q-C time interval, which has been demonstrated to offer enhanced assessment of cardiac contractility. The company also announced FDA 510(k) clearance for expanded BioZ Dx EMR interface capability. The BioZ Dx EMR interface is designed to operate in conjunction with the company's proprietary PC software, BioZport, which automatically sends BioZ ICG clinical data to the customer's local PC network in one of three industry-accepted formats. The data can then be integrated into each patient's electronic medical record.

 June 14, 2024
June 14, 2024