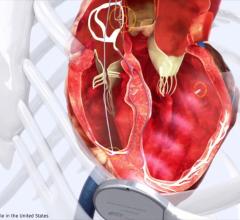
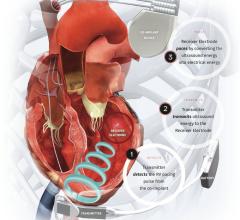
The Acuity steerable left ventricular lead device is designed for cardiac resynchronization therapy defibrillators and cardiac resynchronization therapy pacemakers, both of which treat heart failure.
The product features a deflectable tip for precise placement of the lead even in difficult-to-access branch vessels on the left side of the heart, according to the company.
The FDA cleared the product just a day after Boston Scientific announced FDA officials had lifted all restrictions at the company's Guidant plant in Minnesota.


 July 21, 2025
July 21, 2025