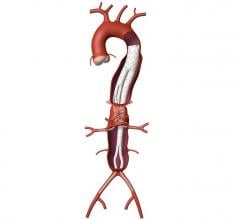
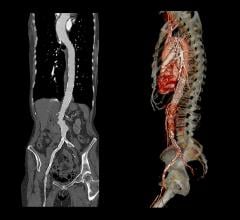
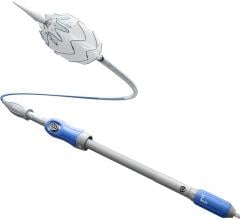
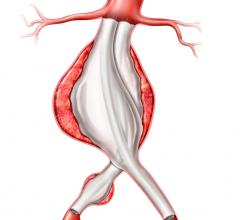
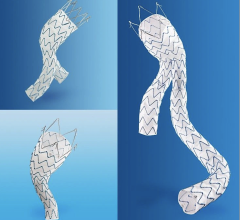
The Relay 250-mm thoracic stent-graft is commercially available in Europe in both straight and taper configurations.
The company received CE approval for Relay in April 2005 for thoracic aortic pathologies including aneurysms, dissections, penetrating ulcers and intramural hematomas. To date, approximately 250 patients have been successfully treated worldwide.
In the U.S., the company is finishing its phase 1 clinical trial and anticipates phase 2 to begin in the third quarter of 2006. Relay is designed for conformance and durability to treat a wide range of thoracic pathologies having both complex tortuous and straightforward morphology. The device's delivery system, the Transport, was designed to accurately and easily deploy the stent-graft in the aortic arch.
The 250-mm stent-graft can enhance short-term outcomes with a single introduction and eliminate the long-term risks associated with joining multiple devices.

 April 26, 2023
April 26, 2023