Step 2/3
Related Content
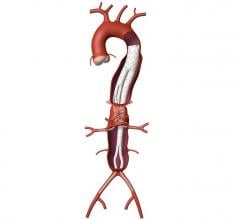
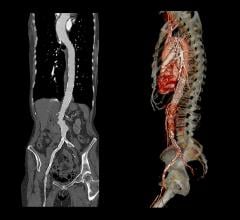
April 26, 2023 — Medtronic today announced its Endurant abdominal aortic aneurysm (AAA) stent graft system continues to ...
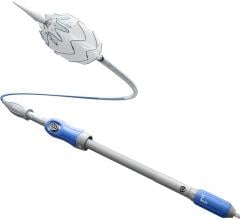
January 12, 2023 — Medtronic announced the first patient enrollment in the ADVANCE Trial, a head-to-head randomized ...
October 9, 2019 — Medtronic plc announced it has received Breakthrough Device designation from the U.S. Food and Drug ...
October 8, 2019 — PQ Bypass Inc. announced it has received full approval of its investigational device exemption (IDE) ...
July 10, 2019 — W. L. Gore & Associates Inc. (Gore) announced the first U.S. implant of the Gore Tag Conformable ...
May 15, 2019 — W. L. Gore & Associates Inc. (Gore) announced the U.S. Food and Drug Administration (FDA) has granted ...
February 14, 2019 — Abdominal aortic stent grafts will remain the largest segment in the global aortic stent graft ...
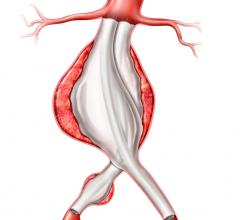
February 7, 2019 — Medtronic plc recently received U.S. Food and Drug Administration (FDA) approval for the Valiant ...
January 7, 2019 — Endologix Inc. announced that in order to ensure optimal outcomes for patients, unrestricted sales and ...

 April 26, 2023
April 26, 2023