March 11, 2009 - With the joint development of the Ziehm NaviPort 3D interface, the medical technology manufacturing companies Ziehm Imaging and Stryker have succeeded in importing 2D and 3D C-arm image data into Stryker’s active infrared navigation systems.
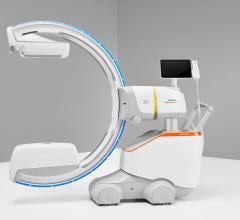
Stryker now supports image import from Ziehm Imaging’s new flat-panel C-arm, the Ziehm Vision FD Vario 3D. Users in the fields of minimally-invasive orthopedic, trauma and spinal surgery particularly benefit from the new level of quality in image-guided navigation.
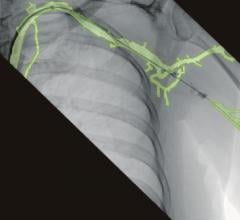
With the new interface there is a link to the active Stryker navigation system with fully automatic registration for image-guided surgery, the companies said. For the first time, doctors are able to integrate intraoperative X-ray images from the latest generation of mobile C-arms into Stryker navigation. The new Ziehm Vision FD Vario 3D flat-detector offers excellent distortion-free 2D image quality as well as efficient intraoperative 3D imaging. The Stryker navigation station can access the most recent fluoroscopic datasets and use them immediately for further procedural interventions. Surgeons and the OR team benefit from an improved visual orientation to patient anatomy during surgery and reduced radiation exposure. Due to these combined technologies, X-ray dose levels can be kept to a minimum. The companies said the imaging capability may also make a postoperative CT scan unnecessary.
The Stryker navigation system is unique in its use of a high-precision, active 3-CCD camera system, which enables much more accurate localization of anatomic structures as well as the instruments deployed in surgery, when compared to traditional solutions. In minimally-invasive surgery, navigation provides the surgeon with a comprehensive overview of the intervention in spite of very small incisions. High-resolution C-arm images contribute to providing precise information to the surgeon, resulting in improved OR safety and improved clinical results.
For more information: www.ziehm.com

 June 20, 2024
June 20, 2024