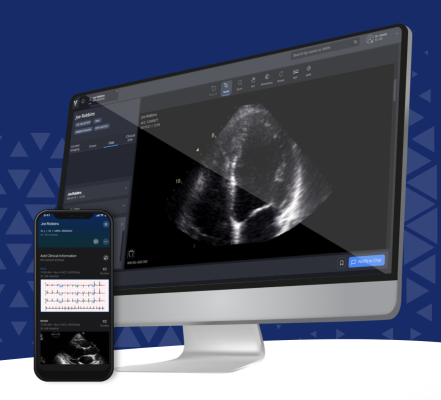
August 15, 2023 — Viz.ai, a leader in AI-powered disease detection and intelligent care coordination, announced it has received a De Novo approval by the U.S. Food and Drug Administration (FDA) for its Viz HCM module, a hypertrophic cardiomyopathy (HCM) artificial intelligence (AI) detection algorithm, creating a new regulatory category for cardiovascular machine learning-based notification software. The deployment of the algorithm is financially supported by a multi-year agreement with Bristol Myers Squibb (NYSE: BMY) announced in March. With the use of Viz HCM, which is integrated into the Viz.ai Platform, more patients with suspected HCM can be identified earlier using AI and triaged for diagnosis and further evaluation.
“Given the high prevalence of patients with suspected HCM who remain undiagnosed, flagging and connecting them quickly to the right providers is critical to improve health outcomes,” said Matthew Martinez, MD, director of sports cardiology and HCM, Atlantic Health System. “The role of artificial intelligence in cardiology is growing exponentially and adding the HCM module to Viz.ai will help increase awareness and reach for HCM patients.”
The Viz HCM module automatically reviews routine electrocardiograms (ECGs) from across a health system to identify suspected HCM cases and notifies the appropriate cardiologists and care team on the Viz mobile application. Clinicians can then easily review the patient’s ECG, coordinate follow-up with an echocardiogram for diagnosis, and use the Viz Echo Viewer to review images and access echocardiogram reports. The HCM module is one of twelve FDA-cleared AI algorithms on the enterprise-wide, clinically validated Viz.ai Platform, which has been shown to increase access to treatments and improve patient outcomes across more than 1,400 hospitals in the US and Europe.
“Hypertrophic cardiomyopathy is a devastating disease that is often undetected until it is too late. The addition of Viz HCM to the Viz.ai Platform aims to improve outcomes for patients with HCM by getting them to the right specialist faster,” said Chris Mansi, MD, CEO and co-founder at Viz.ai. “We are thrilled with this De Novo approval, which establishes the new FDA category of cardiovascular machine learning-based notification software. The ongoing investment of innovative capabilities on our platform is why it continues to be the first choice of leading healthcare systems. With our AI-powered Viz HCM module, we look forward to realizing its promise in expediting detection and care of patients with this common, inherited heart disease.”
For more information: https://www.viz.ai


 February 03, 2026
February 03, 2026 









