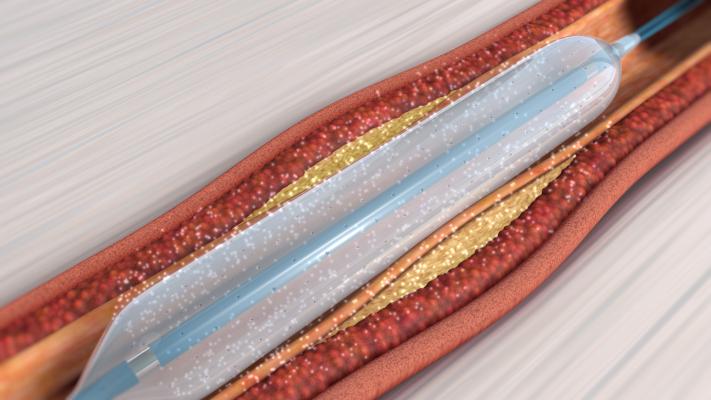
September 20, 2019 — Orchestra BioMed Inc., in partnership with Terumo Corp. announced the company has secured Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA) for its Virtue Sirolimus-Eluting Balloon (SEB) in the treatment of below-the-knee (BTK) peripheral artery disease.
Peripheral artery disease (PAD) affects approximately 8.5 million people in the U.S and is growing dramatically in the elderly population.[1] BTK (infrapopliteal) atherosclerosis is the most common cause of critical limb ischemia (CLI), which is associated with a high rate of amputations and poor survival outcomes.[2] The treatment of BTK atherosclerotic disease is complex, as lesions in these small diameter arteries tend to be diffuse, long and often involve calcification. Currently approved therapeutic options are limited and have been shown to be marginally effective, leaving substantial unmet need for more effective treatment options for BTK atherosclerosis.
“Virtue SEB’s unique design enables delivery of sustained-release sirolimus during angioplasty without the need for coating or permanent implant. This highly differentiated design makes this product the ideal candidate for Breakthrough Device Designation in BTK peripheral artery disease,” said James P. Zidar, M.D., FACC, FSCAI, clinical professor of medicine, UNC Health Systems, physician-in-chief, Heart & Vascular Corporate. “Currently, there is a significant unmet need in the BTK stenosis treatment landscape. The presence of underlying comorbidities renders many patients unsuitable for bypass surgery. Angioplasty with plain balloons, which has been the default endovascular therapy for years, has a low success rate. Adding a proven anti-restenotic agent like sirolimus has the potential to enhance this treatment approach and drive better patient outcomes.”
Virtue SEB is a novel, first-in-class drug/device combination product that delivers a sustained-release sirolimus formulation directly to the artery during balloon angioplasty without the need for a coating. Breakthrough Device Designation is granted to medical devices and device-led combination products that provide the potential for a more effective treatment option for life-threatening or irreversibly debilitating diseases. Manufacturers are then able to offer patients and healthcare providers quicker access to new medical devices by expediting the development, assessment, and review process.
For more information: www.orchestrabiomed.com
References
1. CDC. 2016. Peripheral Arterial Disease Fact Sheet [online] Available at: https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_pad.htm[Accessed 7 August 2019]
2. Fowkes F.G., Rudan D., Rudan I., et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329-40\


 June 13, 2024
June 13, 2024 









