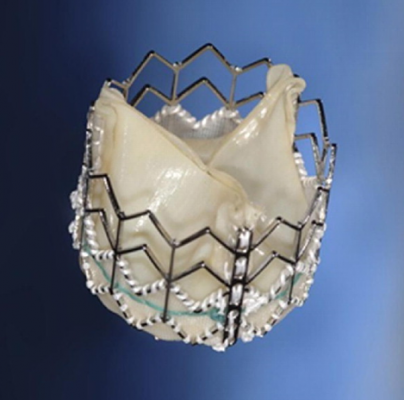
November 16, 2012 — The U.S. Court of Appeals for the Federal Circuit affirmed an April 2010 federal jury decision that Medtronic CoreValve LLC is willfully infringing Edwards Lifesciences' U.S. Andersen transcatheter heart valve patent. The Appeals Court also ordered the trial court to reconsider Edwards' request for a permanent injunction that would prohibit the manufacture and sale of the CoreValve System in the United States.
In addition, the Court affirmed the validity of this patent and the federal jury's verdict awarding an initial payment of $74 million in damages to Edwards, which covers infringement through early 2010. Edwards anticipates that further proceedings by the U.S. District Court for the District of Delaware will require Medtronic to pay substantial additional damages accumulated after the verdict was issued.
"We are very pleased that the appeals court has confirmed that Medtronic CoreValve is illegally using Edwards' transcatheter valve technology. We believe this is a decisive milestone toward final resolution of this matter, given that we have a clear jury verdict that has been affirmed by both the District Court and now the U.S. Court of Appeals," said Aimee S. Weisner, Edwards' corporate vice president, general counsel.
The patent involved in this suit is part of the Andersen family of patents, which relates to a valve prosthesis for implantation by means of a catheter. A petition has been filed with the United States Patent and Trademark Office to extend this patent to 2017.
For more information: www.edwards.com


 December 24, 2025
December 24, 2025 









