April 8, 2024 — People who have had a heart attack or who are at risk for a heart attack and who stopped taking aspirin alongside the P2Y12 inhibitor ticagrelor one month after undergoing percutaneous coronary intervention (PCI) saw a significantly reduced risk of clinically meaningful bleeding with no increased risk of clotting-related adverse events at 12 months compared with patients who continued taking aspirin and ticagrelor for a full year, in a study presented at the American College of Cardiology’s Annual Scientific Session.
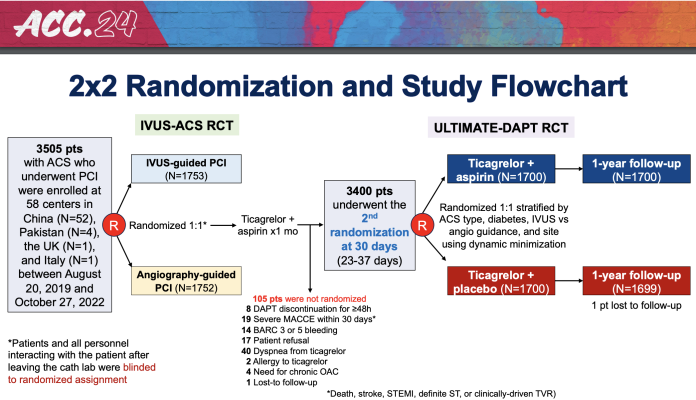
The ULTIMATE-DAPT study is the first placebo-controlled trial designed to examine ticagrelor monotherapy following one month of dual antiplatelet therapy (DAPT) after PCI, a procedure to open blocked arteries. The study focused on patients who underwent PCI for acute coronary syndromes (ACS)—life-threatening conditions which include heart attacks and chest pain caused by decreased blood flow to the heart—with stents containing drugs to prevent further plaque buildup.

Gregg Stone, MD
“In treating a broad range of patients with acute coronary syndromes in the era of contemporary drugeluting stents, among those who were stable after one month of DAPT, continuing treatment with ticagrelor alone reduced bleeding with no increase in adverse ischemic thrombotic events,” said Gregg Stone, MD, professor of cardiology and population health sciences at Icahn School of Medicine at Mount Sinai, New York and co-chair of the trial. “These data suggest that a 12-month duration of DAPT is not only not necessary in most patients with ACS but is harmful.”
Antiplatelet medications reduce clotting-related cardiovascular problems such as heart attacks and strokes by preventing platelets from sticking together. To reduce the risk of such events after PCI, current guidelines recommend that most patients should take two antiplatelet medications—aspirin and a P2Y12 inhibitor—for a full year. However, the bleeding risk associated with antiplatelet medications has fueled efforts to further optimize the duration of post-PCI antiplatelet therapy and the medications used to balance the benefits and risks.
For ULTIMATE-DAPT, researchers enrolled 3,400 patients who experienced no adverse cardiovascular or bleeding events in the first month following PCI for ACS at 58 medical centers in four countries in Asia and Europe. During the first 30 days after PCI, all patients took aspirin and ticagrelor, a potent P2Y12 inhibitor. Participants were then randomly assigned to continue with this same regimen for 11 more months or to switch to ticagrelor and a placebo.
The trial met its two primary endpoints, one assessing efficacy in terms of bleeding risk and the other assessing safety in terms of clotting-related events. The first endpoint, clinically relevant bleeding, occurred in 4.6% of patients assigned to continuing DAPT and 2.1% of patients assigned to take ticagrelor and a placebo, a significant reduction in favor of ticagrelor alone. The second endpoint, a composite of major adverse cardiovascular events and cerebrovascular events, showed no significant difference between groups, with 3.7% of patients who continued DAPT and 3.6% of those taking ticagrelor and a placebo experiencing such events.
The streamlined therapy of treating patients with ACS with ticagrelor alone one month after PCI was equally safe and effective in patients who presented with a heart attack (the highest risk group) or were at risk of a heart attack. Together, these findings suggest that patients who stopped taking aspirin after the first month had a substantially reduced risk of bleeding without any increased risk of thrombotic events, researchers said.
“The next question is how will physicians incorporate these results into their daily practice, and what will guideline committees ultimately do with these data,” Stone said. “I believe these results are very convincing and align with prior studies done without a placebo; hopefully they will impact guidelines and lead to the routine use of only one month of DAPT followed by a potent P2Y12 inhibitor such as ticagrelor in most patients with ACS after successful PCI.”
Since the trial only involved ticagrelor, researchers said that separate studies would be necessary to investigate the safety and efficacy of a similar approach using other P2Y12 inhibitors, such as prasugrel and clopidogrel.
The study was funded by the Chinese Society of Cardiology, the National Natural Scientific Foundation of China and the Jiangsu Provincial & Nanjing Municipal Clinical Trial Project. Stents were supplied by Medtronic Corp. (Minnesota, U.S.) and Microport Medical (Shanghai, China). Study medications were supplied by Yung Shin Pharmaceutical Industrial Co. (Kunshan, China) and Shenzhen Salubris Pharmaceuticals Co., Ltd (Shenzhen, China).
This study was simultaneously published online in The Lancet at the time of presentation.
For more information: www.acc.org


 July 31, 2024
July 31, 2024 









