April 7, 2024 — An estimated 1 in 5 U.S. adults—and more than 2 in 5 adults aged 60 years and older—have elevated triglycerides, also known as hypertriglyceridemia, putting them at an increased risk for heart attacks and stroke. However, few treatments are currently available for this patient population, until now. A new investigational drug called olezarsen holds promise for substantially reducing triglyceride levels without significant adverse effects, according to a study presented at the American College of Cardiology’s Annual Scientific Session.
It is well known that high levels of low-density lipoprotein (LDL) cholesterol, known as the “bad” cholesterol, heighten cardiovascular risk. It is less well known that high levels of triglycerides (defined as over 150 mg/dL) are also bad for the heart. Triglycerides store unused calories and provide energy to the body. As with elevated LDL-cholesterol, high levels of triglycerides and the lipid particles on which they are carried in the blood can contribute to the formation of “plaques” in the arteries that impede blood flow and can lead to heart attacks and strokes. Severely elevated triglyceride levels (>500 mg/dL) can also cause pancreatitis, an inflammatory process in the pancreas that can be life threatening.

Brian Bergman, MD
“Treatments to reduce high triglycerides are an unmet clinical need,” said Brian Bergmark, MD, of the Thrombolysis in Myocardial Infarction (TIMI) Study Group at Brigham and Women’s Hospital and Harvard Medical School and the study’s principal investigator. “Based on our study’s results, we can say that the drug worked and appeared to be safe. We tested two doses of olezarsen, and both reduced triglyceride levels equally well.”
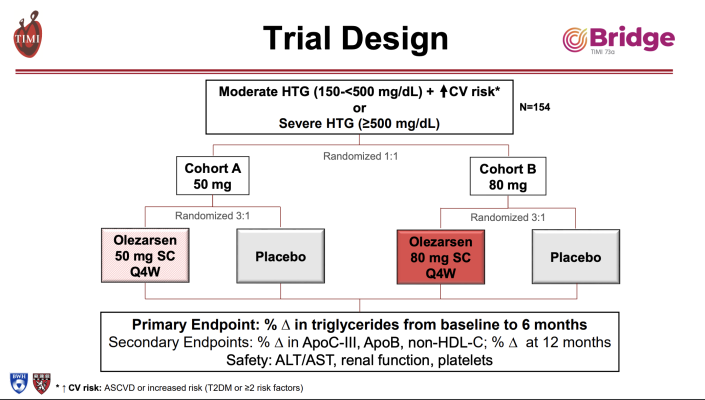
Olezarsen blocks the body from making apolipoprotein C-III (apoC3), which plays a key role in triglyceride levels. A previous study found that olezarsen significantly reduced levels of both triglycerides and apoC3 in patients with moderate hypertriglyceridemia. The goal of the current study, known as BRIDGE-TIMI 73a, was to compare the triglyceride-lowering effects of two doses of olezarsen in patients with elevated triglyceride levels who were already receiving standard-of-care treatment for their lipids.
A total of 154 patients (median age 62 years, 42% women) with high triglyceride levels and other risk factors for cardiovascular disease participated in the study at 24 sites in the U.S. and Canada. Patients’ median triglyceride level was 242 mg/dL. Patients were randomly assigned to receive an injection of 50 mg or 80 mg of olezarsen or a placebo every four weeks for up to 49 weeks. The study was designed so that neither patients nor their clinicians, the investigators or the experts who assessed patient outcomes knew which patients were receiving the drug and which the placebo until after the study was completed. Patients were followed for one year of treatment.
The study’s primary endpoint was the percentage change in triglyceride levels at six months. Secondary endpoints included the percentage change in triglyceride levels at 12 months and percentage changes in non-HDL cholesterol and another blood protein, apolipoprotein B (apoB) that, when elevated, can also signal elevated risk for heart disease or stroke.
Results showed that the 50 mg dose of olezarsen reduced triglyceride levels by 49% and the 80 mg dose reduced triglyceride levels by 53%. Patients taking the 50 mg dose saw an average reduction of 64% in levels of apoC3, while those on the 80 mg dose saw an average reduction of 74% in apoC3. Levels of apoB were reduced by about 18% on both doses. All reductions in lipid levels were maintained for 12 months. Clinically significant adverse effects such as abnormalities affecting the kidneys, liver or platelets (blood cells that help the blood to clot) were uncommon.
In terms of the triglyceride-lowering effect alone, the difference between the 50 mg and 80 mg doses of olezarsen was relatively small, Bergmark said. However, the 80 mg dose resulted in a larger reduction in apoC3 than the 50 mg dose. In addition, he said, the reduction in levels of apoB is of interest because this protein is found on all of the lipid particles that contribute to cholesterol buildup in the arteries.
“If you actually want to reduce a patient’s risk for a heart attack or stroke, you would like to see a reduction in apoB, and we did see that in this study, which is very encouraging,” he said.
Limitations of the study are its relatively small size, limited follow-up period and inclusion of few patients with severe hypertriglyceridemia (triglyceride levels of 500 mg/dL or higher). Bergmark said, phase 3 studies of olezarsen, including in patients with severe hypertriglyceridemia, are underway and are expected to help address these remaining questions.
The study was funded by Ionis Pharmaceuticals, Inc., which manufactures olezarsen.
This study was simultaneously published online in the New England Journal of Medicine at the time of presentation
For more information: www.acc.org


 July 31, 2024
July 31, 2024 









