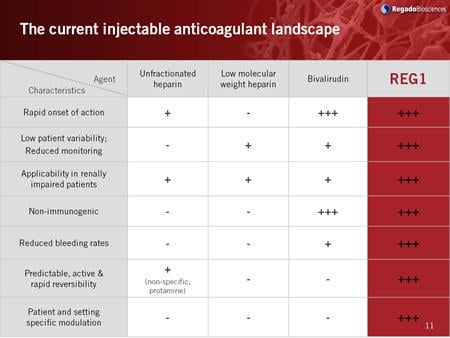
September 10, 2009 – Regado Biosciences said this week it enrolled the first patient in a phase 2b, randomized, partially-blinded, multicenter, active-controlled, dose-ranging study of its REG1 anticoagulation system.
REG1 comprises the selective factor IXa inhibitor, RB006, and its specific active control agent, RB007. The trial, called RADAR, will assess the safety, efficacy, and pharmacodynamics of REG1 compared to unfractionated heparin or low molecular weight heparin in subjects with acute coronary syndromes (ACS). Regado recently successfully completed a phase 2a study of REG1 in stable coronary artery disease patients undergoing elective PCI.
The primary objectives of RADAR will be to determine the clinically acceptable dose range of the specific active control agent, RB007, which can be used to reliably partially or totally reverse the anticoagulant effect of RB006 while reducing bleeding in comparison to heparin, and to determine the pharmacodynamics of the REG1 anticoagulation system in subjects intended for cardiac catheterization within 24 hours who are admitted for ACS-unstable angina and myocardial infarction without ST-segment elevation (UA/NSTEMI).
RADAR, led by principal investigator John H. Alexander, M.D., MHS, FACC, Duke University, will enroll 800 subjects at approximately 75 centers around the world. Participating countries include the United States, Canada, Poland, France, Germany, Netherlands, and Belgium. More information about RADAR can be found at www.clinicaltrials.gov. The identifier for this trial is NCT00932100.
The anticoagulant system REG1 consists of two parenteral agents both administered by IV bolus, the first being a potent highly selective Factor IXa inhibitor (RB006) and the second being its complementary active control agent (RB007). RB007 can be used to selectively completely or partially reverse the anticoagulant effect of RB006. REG1 is intended for application in arterial thrombosis applications, initially in Acute Coronary Syndrome patients undergoing Percutaneous Coronary Intervention. REG2, Regado's second product candidate, consists of a subcutaneously administered formulation of RB006 paired with the IV bolus formulation of RB007. REG2 is intended for use in venous thrombosis indications.
For more information: www.regadobio.com


 January 05, 2026
January 05, 2026 









