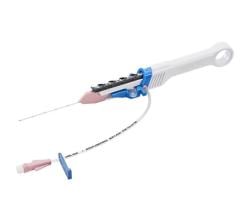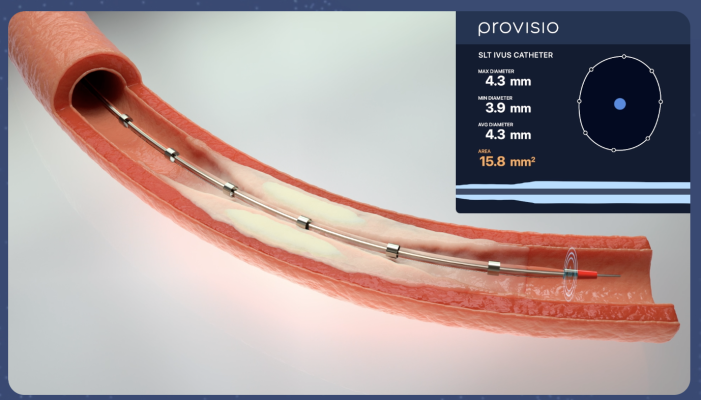
April 25, 2024 — Provisio Medical announced FDA 510(k) clearance of the Provisio SLT IVUS System. Sonic Lumen Tomography (SLT) technology addresses a critical unmet need for vascular specialists by providing automatic, real-time, accurate, numeric measurements of the flow lumen of blood vessels without the complexities of image interpretation. Provisio Medical's catheter is the world's first integrated intravascular imaging and support crossing catheter and enables vessel lumen measurement and visualization simultaneously with guidewire support and delivery of radiopaque contrast agents.
It has been demonstrated repeatedly that the current standard-of-care of using angiographic information by itself is insufficient to accurately assess vessel size1 in the approximately 20 million people in the U.S. with peripheral vascular disease2. The use of intravascular imaging has been shown to improve accuracy of vessel sizing and thereby improve clinical outcomes3. By incorporating its technology into a front-line support crossing catheter, Provisio simplifies the acquisition and presentation of vessel sizing data without changing the physician's workflow, while potentially reducing the time for data acquisition, use of contrast media, and exposure to radiation.
"Clinical outcomes in peripheral vascular disease have consistently been shown to benefit from accurate intravascular measurements, yet adoption has been limited by the additional procedure time and training required to interpret images" noted S. Eric Ryan, MD, Chief Executive Officer. "Thanks to the ease-of-use of SLT IVUS, which can be incorporated more efficiently in the peripheral vascular workflow, we believe there is the possibility of increased adoption and therefore improved outcomes for many more patients with potentially devastating peripheral vascular disease."
The Provisio SLT IVUS System consists of the SLT IVUS P1 System and the SLT IVUS Support Crossing Catheter. The SLT IVUS Support Crossing Catheter is an over-the-wire intravascular ultrasound catheter with an ultrasound transducer array at the distal end that also functions as a support crossing catheter. The information from the ultrasound signal is used similarly to sonar technology to measure vessel dimensions in real-time and visualize the peripheral vessel's flow lumen.
For more information: www.provisiomedical.com
References:
1 Pliagas G, Saab F, Stavroulakis K, Bisdas T, Finton S, Heaney C, McGoff T, Hardy K, Adams G, Mustapha JA. Intravascular Ultrasound Imaging Versus Digital Subtraction Angiography in Patients with Peripheral Vascular Disease. J Invasive Cardiol. 2020 Mar;32(3):99-103.
2 Yost, Mary L., The True Prevalence of PAD and the Economics of Major Amputation, Endovascular Today, May 2021
3 Secemsky EA, Parikh SA, Kohi M, Lichtenberg M, Meissner M, Varcoe R, Holden A, Jaff M, Chalyan D, Clair D, Hawkins B, Rosenfield K. Intravascular ultrasound guidance for lower extremity arterial and venous interventions. EuroIntervention. 2022 Sep 20;18(7):598-608. doi: 10.4244/EIJ-D-21-00898. PMID: 35438078; PMCID: PMC10331977.


 January 29, 2026
January 29, 2026