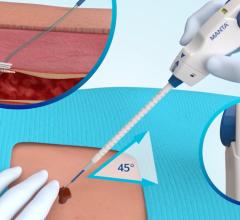
ProGlide Medicated Suture Closure Device
Related Content
Oct. 7, 2025 — Nobles Medical Technology II, a provider of cardiovascular closure solutions, has announced that the U.S ...
April 16, 2024 — Vivasure Medical, a company pioneering novel fully absorbable technology for percutaneous vessel ...
September 16, 2022 — Teleflex Incorporated, a leading global provider of medical technologies, announced that Dr. Magnus ...
November 9, 2021 — Results from the largest randomized trial available comparing different closure device strategies ...
July 15, 2021 — Vivasure Medical announced its development program for PerQseal Blue, a sutureless and fully ...
August 17, 2020 — Veryan Medical announced it will support Vasorum in the commercialization of the Celt atrial closure ...
Ashish Pershad, M.D., chief of interventional cardiology, Banner University Medical Center, Phoenix, explains the trend ...
April 3, 2019 — Essential Medical Inc. received U.S. Food and Drug Administration (FDA) clearance for its large bore ...
December 20, 2018 — Cardiva Medical Inc. announced the company has received premarket approval (PMA) from the U.S. Food ...
November 21, 2018 – Vivasure Medical Ltd. recently announced the European launch of the PerQseal closure device for ...


 October 07, 2025
October 07, 2025