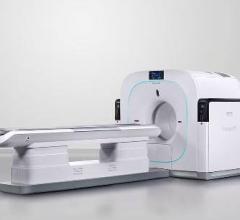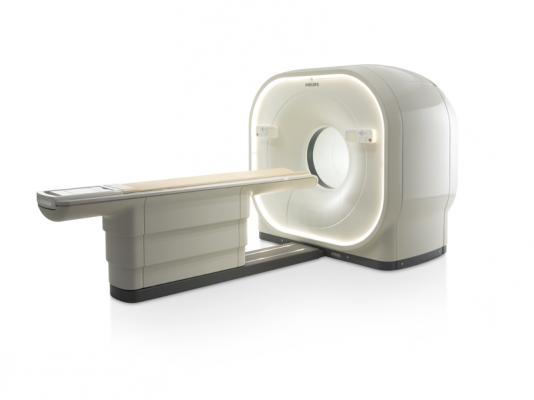
June 12, 2014 — Philips Healthcare recently introduced Vereos PET/CT, the first digital PET/CT (positron emission tomography/computed tomography) scanner, at the 2014 annual meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI) in St. Louis. In addition to Vereos, Philips showcased a selection of molecular imaging solutions designed to deliver high image quality, critical clinical information and greater connectivity.
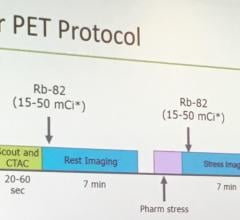
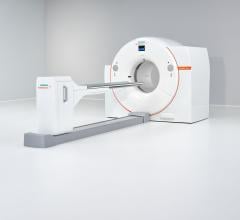
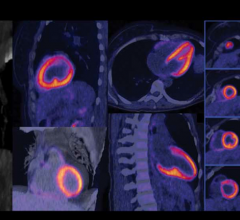
The Vereos PET/CT uses digital silicon photomultiplier detectors instead of traditional analog detectors, creating a step change in performance that includes approximately two-times increase in sensitivity gain, volumetric resolution and quantitative accuracy compared to analog systems. These improvements can ultimately be translated into high image quality, increased diagnostic confidence, improved treatment planning and faster workflows.
"From connected devices to data-driven insights, the pace of imaging innovation is allowing us to see more," said Gene Saragnese, chief executive officer, Imaging Systems, Philips. "Molecular imaging technology is leading the way with digital detection and advanced visualization built on insights from those at the heart of care."
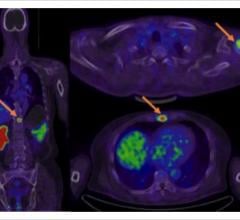
Visitors to the Philips booth at SNMMI had the opportunity to interact with Vereos digital PET/CT clinical images and technology using engaging 3-D visualization tools. The experience provided clinicians an understanding of how Vereos can be used to deliver customized care to meet their most pressing needs.
During the SNMMI annual meeting, Philips also hosted a series of three "Innovation Talks" in its booth theater area, spotlighting customers, scientists and industry thought leaders who discussed topics related to the current and future landscape for digital PET, patient-centered imaging and the importance of creating customized workflows to help manage patient care and radiation dose.
Other advanced molecular imaging technology solutions showcased include”
- iPatient: An advanced platform with an intuitive interface, iPatient helps to facilitate patient-centered imaging, allows for efficient communication between the CT system and injector, and aids in the delivery of appropriate contrast dose and consistent image quality.
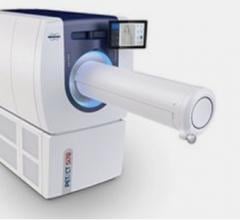
- BrightView XCT: A SPECT/CT system designed entirely for nuclear medicine, BrightView XCT enables low patient dose levels, high-resolution localization and high-quality attenuation correction, for smart assessments and short exams with the patient's comfort in mind.
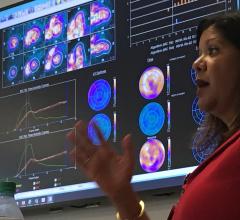
- IntelliSpace Portal: Part of Philips' family of clinical information systems within Philips Healthcare’s Informatics Solutions and Services Group, the IntelliSpace Portal offers extensive clinical coverage, streamlined workflow and continuous evolution of advanced visualization and analysis capabilities within a single solution.
For more information: www.philips.com/snmmi


 June 23, 2025
June 23, 2025