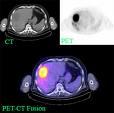
February 17, 2009 - The total PET and PET/CT patient studies increased only 4 percent, from 1,457,400 to 1,520,800, compared to estimates for 2007 provided by respondents to a 2008 survey by IMV Medical Information Division, representing a slowdown in procedure growth.
Despite the 2008 figures represent a 35 percent increase over the same survey by IMV done in 2005, which estimated 1,129,900 patient studies were performed using PET or PET/CT, the recent slowdown may be caused by the Deficit Reduction Act (DRA) of 2005, indicated Lorna Young, senior director, Market Research at IMV.
“The estimated 1,520,800 clinical PET patient studies performed in 2008 represent a 35% increase over IMV’s 2005 estimate of 1,129,900 patient studies, for an average annual growth rate of 10.4 percent over the three-year period,” said Young. “With the advent of the Deficit Reduction Act (DRA) of 2005, related legislation against self-referral for standalone imaging centers, the increased scrutiny of third party insurers in their preauthorization processes and the reduction in the availability of capital, the market for PET purchases may continue to slow over the next few years.”
The report just released by IMV accounted for PET studies performed in 2,000 U.S. hospital and non-hospital sites, using fixed or mobile PET/CT or PET scanners.
Young added, “The PET market for fixed PET imaging units is still relatively early in its adoption cycle, as over half of the PET sites use a mobile service provider, while over 900 sites own one or more fixed PET or PET/CT scanners. A market forecast scenario presented in the report shows that over the next few years, 55 percent of the PET scanner demand will come from first buyers, who currently use mobile services, 30 percent will be replacement buyers and 15 percent will be additional buyers.”
The survey also noted that of the patient studies performed on PET or PET/CT scanners, 94 percent are for oncology applications, and 6 percent are for cardiology and neurology applications.
For more information: www.mvinfo.com


 November 17, 2025
November 17, 2025 






![Phase III clinical trial of [18F]flurpiridaz PET diagnostic radiopharmaceutical meets co-primary endpoints for detecting Coronary Artery Disease (CAD)](/sites/default/files/styles/content_feed_medium/public/Screen%20Shot%202022-09-13%20at%203.30.13%20PM.png?itok=2w6OoNd6)
