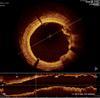
Image created through optical coherence tomography (OCT) of the coronary arteries.
March 29, 2010 - Optical coherence tomography (OCT) not only has the potential to identify vulnerable plaques, but in countries like Japan, it is already a standard follow up to stenting procedures to determine if the stent has effectively widened the affected artery and the area is free of blood clots.
Massachusetts General Hospital (MGH), together with a coalition of 20 international sites in five countries, will create the largest registry of patients who have had OCT of the coronary arteries. OCT is an intravascular imaging technology visualizes the microscopic characteristics of a vulnerable plaque - such as a large lipid pool covered by a thin fibrous cap – by creating extremely high-resolution images from within the coronary artery. Using near-infrared light, OCT bounces light off the vessel wall and collects details down to 10 microns, a resolution at least 20 times better than computed tomography. The resulting image of the coronary artery, captured in a matter of seconds, offers a clear depiction of the plaque's architecture. This technology is especially unique as standard imaging technologies are not able to identify the microscopic characteristics of vulnerable plaques.
Twenty sites in five countries – Australia, China, Japan, Korea, and the United States – will collect data from 3,000 patients who have had OCT of the coronary arteries during a cardiac catheterization procedure and follow them for five years. MGH researchers will gather the data in a central database. Researchers hope the data will help determine the efficacy of OCT in identifying vulnerable plaques in patients as well as its benefits as a follow-up procedure to stent placement.
The Massachusetts General Hospital OCT Registry will include the more than 10,000 OCT cases performed in 2009. Researchers launched the registry was launched during the first MGH OCT Registry Symposium held March 13 in Boston. The symposium, sponsored by LightLab Imaging Inc., was the first gathering of OCT experts held in the United States and was a forum for physicians to share the latest work in the field. International sites will begin enrolling patient in the MGH OCT Registry in June.
Enrollment in the United States will begin pending clearance of OCT by the U.S. Food and Drug Administration.


 October 09, 2025
October 09, 2025