May 4, 2015 — Results from Lantheus Medical Imaging Inc.’s first Phase 3 study of flurpiridaz F 18 for myocardial perfusion imaging (MPI) were presented at the International Conference on Nuclear Cardiology and Cardiac CT (ICNC12) in Madrid, Spain. The oral presentation was made by Jamshid Maddahi, M.D., professor of medicine (cardiology) and molecular & medical pharmacology (nuclear medicine), David Geffen School of Medicine at UCLA, in the “Important Clinical Trials and Registries in Nuclear Cardiology” session of ICNC12.
“Analysis of the first Phase 3 clinical study has confirmed the value of this novel agent as compared with standard SPECT imaging for the diagnosis of coronary artery disease,” said Maddahi, the principal investigator of the study. “The results of this study provide additional evidence for flurpiridaz F 18 as a very important potential new agent for the diagnosis and characterization of coronary artery disease.”
The Phase 3 results for flurpiridaz F 18 are from a multicenter, international study enrolling 795 subjects with known or suspected coronary artery disease (CAD) and scheduled for coronary angiography and single photon emission computed tomography (SPECT) imaging. Flurpiridaz F 18 is an investigational positron emission tomography (PET) MPI agent in late stage clinical development.
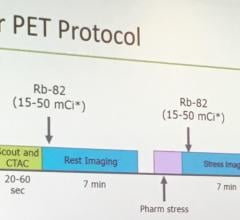
In the first Phase 3 study, subjects underwent flurpiridaz F 18 PET MPI and SPECT MPI studies, with coronary angiography used as the truth standard for each. The currently available PET MPI agents do not generally allow sufficient time for exercise stress testing and are thus limited to pharmacological stress testing. With a 110-minute half-life, flurpiridaz F 18 can be easily used in association with treadmill exercise stress testing, which in itself can give a clinician valuable observational data about a patient’s condition to complement the imaging results.
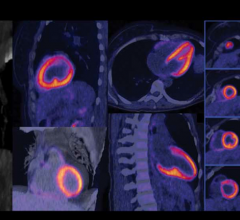
The Phase 3 study showed that in a well-controlled setting, flurpiridaz F 18 imaging consistently performed in a balanced manner in identifying disease (sensitivity) and ruling out disease (specificity) when compared to coronary angiography. Unlike flurpiridaz F 18, SPECT imaging results were skewed with low sensitivity and high specificity. The Phase 3 study’s two pre-specified primary endpoints required a comparison of the balanced flurpiridaz F 18 sensitivity and specificity results and the skewed SPECT sensitivity and specificity results. In this comparison, flurpiridaz F 18 far exceeded SPECT for sensitivity but did not meet the non-inferiority threshold for specificity, implying a substantial and unexpected under-diagnosis of CAD with SPECT imaging in the trial.
The Phase 3 study also compared diagnostic accuracy of flurpiridaz F 18 versus SPECT imaging, showing statistical significance in the overall population and in those populations of special clinical interest, including women and obese subjects (body mass index of 30 or greater). Superiority over SPECT was further demonstrated by comparing the area under the receiver operator curve (ROC) for CAD diagnosis. Superiority over SPECT for diagnostic accuracy in the area under the ROC curves was particularly pronounced in women, obese subjects and subjects with multivessel disease.
Importantly, radiation exposure associated with flurpiridaz F 18 imaging was reduced to approximately 50 percent of that associated with SPECT MPI. Even with reduced radiation, a significantly higher percentage of images were rated as either excellent or good quality with flurpiridaz F 18 imaging, compared to SPECT imaging for stress images and rest images, leading to greater diagnostic certainty of interpretation — namely, the percentage of cases with definitely abnormal or definitely normal interpretation. No drug-related serious adverse events were observed.
Based on the results of the first Phase 3 study, Lantheus redesigned the protocol for its second Phase 3 study, including different primary endpoints — namely, the performance of flurpiridaz F 18 on its own merit versus coronary angiography as the truth standard. The U.S. Food and Drug Administration has granted a Special Protocol Assessment in connection with the new second Phase 3 study, and Lantheus is in active discussions with prospective partners for the further development and commercialization of this promising candidate.
For more information: www.lantheus.com


 June 23, 2025
June 23, 2025