February 4, 2011 – Researchers at the National Institutes of Health's (NIH) Undiagnosed Diseases Program (UDP) have identified the genetic cause of a rare and debilitating vascular disorder not previously explained in the medical literature. The adult-onset condition is associated with progressive and painful arterial calcification affecting the lower extremities, yet spares patients’ coronary arteries. The new disease finding was published in the New England Journal of Medicine.
The rare arterial condition caused by calcium buildup in arteries below the waist and in the joints of patient’s hands and feet has been observed in nine individuals from three unrelated families, who are the only people known to have the disorder. The researchers refer to the condition as arterial calcification due to CD73 deficiency, or ACDC. Although symptoms of the disorder include leg and joint discomfort, medical evaluations ruled out rheumatoid arthritis or other joint-related problems. Genetic analyses suggested a novel disorder and pinpointed the cause of the condition as mutations, or variants, in the NT5E gene.
"This is the first novel disease discovery identified through the collaborative and interdisciplinary approach employed by clinical researchers in the NIH Undiagnosed Diseases Program," said NIH Director Francis S. Collins, M.D., Ph.D. "This disorder previously baffled the medical field and evaded diagnosis when conventional methods were used."
The NIH clinical researchers examined members of two families with the arterial calcification disorder as part of the UDP and identified a third case outside the country. Seven medical cases like those described in this study have been reported in medical journals during the past century, but these previous studies did not include any insights about the molecular basis of the disorder.
“This study shows that genomic tools are a powerful ally in our search to discover and understand rare diseases,” said Eric D. Green, M.D., Ph.D., director of the National Human Genome Research Institute.
The UDP program, entering its third year, receives medical referrals from around the country when cases challenge the diagnostic know-how and resources of the medical community. Patients enrolled in the program undergo extensive medical diagnostic testing and evaluation at the NIH Clinical Center in Bethesda, Md.
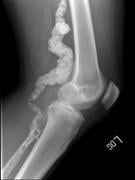
Members of two of the three families reported in this study were enrolled and examined as part of the UDP. The patients experienced pain and cramping in the calves, thighs, buttocks and feet due to poor circulation. Magnetic resonance imaging (MRIs) and X-rays of the patients’ vasculature indicated calcium deposits in artery walls. For one of the patients, advancement of the condition had been treated with surgeries to reroute blood flow through alternate vessels, as well as a joint amputation in the foot. Peripheral blood vessels compensate to some extent for diminished blood flow in affected arteries.
In one of the families with five affected siblings, clinical researchers suspected a recessive inheritance, in which offspring receive two copies of a gene variant that produces disease symptoms only when combined. The researchers analyzed DNA from all members of the family to compare the parents’ DNA to that of their affected children. This allowed researchers to detect genomic regions where the siblings’ DNA contained two copies of a particular DNA segment compared to their parents’ DNA, which contained just a single copy.
The comparison revealed one such region, which the researchers subsequently analyzed for sequence variants not present in a population of 200 unaffected people. The siblings all had the same variant in a gene called NT5E. This gene normally makes the CD73 protein, which produces a small molecule, adenosine, which protects the arteries from calcifying. The researchers also detected variants in NT5E in all the other affected patients in the study. The researchers performed laboratory tests to characterize the molecular basis of the arterial calcification disorder and to validate various molecular activities in cells with NT5E variants.
"We were able to illustrate that elevated activity of a key enzyme in tissue calcification, called TNAP, was due to the lack of extracellular adenosine," said lead author Cynthia St. Hilaire, Ph.D., a postdoctoral fellow at the National Heart, Lung and Blood Institute (NHLBI). In turn, TNAP degrades an inhibitor of calcification, called pyrophosphate. The researchers therefore tied the elevation in TNAP activity with increases in arterial calcification. They also suggest that the location of calcification may correspond to the distribution of specific adenosine receptors in the body.
"Vascular calcification often results from poor diet and lack of exercise," said co-author William A. Gahl, M.D., Ph.D., NHGRI clinical director and director of the NIH Undiagnosed Diseases Program. "The calcium buildup in arteries of our patients, however, arises because the systems to inhibit it are not working in their cells. We hope that an understanding of this faulty mechanism will guide us in providing helpful treatments for these patients."
"The diagnosis of this faulty gene is the first molecular description of this disorder," said Manfred Boehm, M.D., lead senior author and NHLBI investigator. "In addition to providing insight for this unique patient group and their physicians, the study has placed this condition among disorders it resembles, adding to our knowledge of vascular biology."
For more information: rarediseases.info.nih.gov/undiagnosed


 January 05, 2026
January 05, 2026 









