May 31, 2022 — Findings from the XARELTO (rivaroxaban) Phase 3 COMPASS Long-Term Open Label Extension (LTOLE) study and the XARELTO in Combination with Acetylsalicylic Acid (XATOA) registry were published in the European Society of Cardiology’s (ESC) European Heart Journal, Cardiovascular Pharmacotherapy. Additionally, the XATOA registry was presented at the American Congress of Cardiology’s 71st Annual Scientific Session (ACC.22). These studies provide further evidence supporting the role of dual pathway inhibition (DPI) with the XARELTO vascular dose (2.5 mg twice daily plus aspirin 100 mg once daily) in patients with CAD and/or PAD.
The COMPASS LTOLE study found that continued treatment with XARELTO 2.5 mg twice daily plus aspirin 75 to 100 mg once daily for up to three years was associated cp-318722v1 with similar or lower incidence rates for major cardiovascular events (MACE) - cardiovascular (CV) death, stroke, or myocardial infarction (MI) - and for bleeding than those seen during the randomized treatment phase. Separately, the XATOA registry provides real-world evidence of the benefit of DPI with the XARELTO vascular dose in patients with CAD and/or PAD.
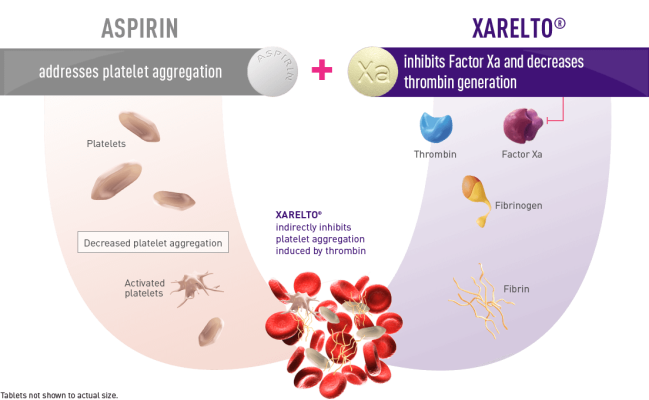
Patients with CAD and/or PAD are at risk of secondary thrombotic events associated with CV disease including stroke, MI, ischemic limb events, and CV-related death.1 Antiplatelet therapies, such as aspirin, are used to help prevent thrombosis by inhibiting platelet aggregation. 1 Antithrombotic therapy is often recommended in addition to an antiplatelet therapy (commonly aspirin) – known as DPI – to further reduce residual risk of CV events in certain patients with CAD and/or PAD. 2 The XARELTO vascular dose (2.5 mg twice daily plus aspirin 100 mg once daily) is the first and only approved treatment for CAD and PAD using a DPI approach to target both clotting mechanisms – thrombin generation and platelet activation.
“What we saw initially in COMPASS, and now again with its long-term open label continuation study, is that dual-pathway inhibition with XARELTO plus aspirin can significantly reduce the underlying thrombotic risk of cardiovascular events in patients with CAD and/or PAD,” said James F. List, M.D., Ph.D., Global Therapeutic Area Head, Cardiovascular, Metabolism, and Retina, Janssen Research & Development, LLC. “Additionally, through the real-world XATOA registry, we’ve now uncovered, for the first-time, which types of patients are most often selected for and may benefit from dual pathway inhibition. These data provide practical, clinical insights to those who treat these difficult conditions.”
COMPASS LTOLE results support long-term use of XARELTO plus aspirin
COMPASS, the largest clinical trial of XARELTO to date with 27,395 patients, showed that the XARELTO vascular dose reduced the risk of major CV events by 24 percent in patients with chronic CAD and/or PAD. Of the patients originally randomized in COMPASS, 12,964 were subsequently enrolled in the LTOLE study to investigate the long-term use of XARELTO plus aspirin for up to three years.
The findings showed that XARELTO 2.5 mg twice daily plus aspirin 75 mg to 100 mg once daily was associated with a primary outcome event rate (CV death, stroke or MI) of 2.35 (95% CI 2.11-2.61 per 100 patient years; n=353), which was similar to the rate observed during the randomized treatment phase (2.18 [95% CI 1.97-2.41] per 100 patient years; n=379). Major bleeding rates during LTOLE had an incidence rate of 1.01 [95% CI 0.86-1.19], whereas the randomized phase showed major bleeding of 1.67 [95% CI 1.48-1.87] per 100 patient years. The COMPASS randomized clinical trial results were previously published in the New England Journal of Medicine in 2017, which confirmed the safety profile of XARELTO plus aspirin.
“When first presented, the COMPASS results represented a real breakthrough in CAD and PAD, as they confirmed the XARELTO vascular dose is effective in helping to prevent the detrimental cardiovascular events that often occur in these patients,” said Professor John Eikelboom*, McMaster University in Hamilton, Canada, and lead author of the COMPASS and LTOLE studies. “It’s extremely reassuring to see that these benefits continue long-term, as observed in our open label extension study.”
The COMPASS LTOLE study was not randomized and did not include a control group. Only 47 percent of the original COMPASS cohort entered LTOLE, thereby introducing potential selection and survival biases. Patients who were entered into LTOLE are not directly comparable to those who were originally randomized in the COMPASS trial as the two cohorts are overlapping and patients enrolled in LTOLE are several years older.
Real-world XATOA registry reconfirms findings from COMPASS
The XATOA registry investigated the clinical characteristics in CAD and/or PAD patients prescribed DPI using XARELTO 2.5 mg plus aspirin and reported the clinical outcomes and bleeding rates in clinical practice compared to the COMPASS randomized trial. The XATOA registry helps provide clinicians additional information on which types of patients may benefit from DPI therapy in clinical practice.
The full analysis set consisted of 5,532 patients with CAD and/or PAD who received at least one dose of DPI with XARELTO 2.5 mg and aspirin. Of the patients in the full analysis set, 4,022 (72.7%) had CAD, 3,258 (58.9%) had PAD, and 1,748 (31.6%) cp-318722v1 had both CAD and PAD. The average observation period in the full analysis set was 15±6 months and 79.1% (n = 4,374) were followed up for more than 12 months.
The results found the most frequently reported reason for initiating DPI was the presence of existing, worsening or newly diagnosed risk characteristics (n = 4753, 85.9%). The rates of MACE were similar to those in the COMPASS trial (2.26 vs 2.18 per 100 patient years) and rates of major adverse limb events (MALE) were higher (3.57 vs 0.19), consistent with the greater proportion of patients with PAD in the study. The study also showed that major bleeding occurred at a rate of 0.95 (per 100 patient years) compared to 1.7 (per 100 patient years) in the COMPASS trial.
“For the first time, we’ve uncovered which patient characteristics go into a physician’s decision making for prescribing dual-pathway treatment to help reduce the risk of major cardiovascular events,” said Professor Keith Fox**, University of Edinburgh, and lead author of the XATOA registry. “In the XATOA registry, patients who received treatment with XARELTO plus aspirin had outcomes consistent with the COMPASS study. These real-world findings help support the use of DPI in patients at increased risk of vascular events.”
Patients enrolled in the XATOA registry were screened consecutively to reduce selection bias and measures were taken to ensure that the enrolled patients were representative of the population of each study site. As outcomes were collected in different ways in XATOA and COMPASS, differences between outcomes in the studies are inherent.
About CAD and PAD
Cardiovascular diseases (CVD), including coronary artery disease (CAD) and peripheral artery disease (PAD), account for around 1 in every 3 deaths worldwide.3 Despite advances in management, CVDs continue to place a significant burden on healthcare, social care, and financial systems.4 CAD, or ischemic heart disease, is the most common type of heart disease, affecting approximately 18.2 million adults in the U.S. 5 PAD is a common chronic circulatory condition that causes blood vessels to narrow, thereby reducing blood flow to the limbs, and most often the legs.6 It is a disease which often goes undiagnosed and undertreated.7 In the U.S., PAD affects an estimated 20 million adults, yet only approximately 8.5 million are diagnosed.8,9
About the XATOA Study
XATOA was an international, multi-center, prospective, single-arm study designed to provide insights into the clinical characteristics of patients selected for DPI with CAD, PAD or both and their clinical outcomes and bleeding rates in clinical practice. 5,532 patients were included, and most were treated only with aspirin prior to enrollment.
Clinical outcomes of interest included major adverse cardiovascular events (MACE), and major adverse limb events (MALE). The safety outcome was the International Society on Thrombosis and Haemostasis (ISTH) major bleeding. Outcome definitions were harmonized with those of the COMPASS study to allow comparisons of the data.
About the COMPASS and COMPASS LTOLE Studies
In the double-blind part of the Phase III COMPASS study the vascular dose of XARELTO (2.5 mg twice daily plus aspirin 100 mg once daily) reduced the risk of stroke, myocardial infarction, or cardiovascular death and all-cause mortality in patients receiving a high standard of risk factor management for CAD and/or PAD. The treatment resulted in a relative risk reduction of 42 percent in stroke and 22 percent in cardiovascular death compared with aspirin 100 mg once daily alone. Bleeding rates were low, and while major bleeding increased, notably there was no significant increase in intracranial or fatal bleeding.
Of the patients originally randomized in COMPASS, 12,964 were subsequently enrolled in the open-label extension part, from 455 sites in 32 countries. All patients received XARELTO 2.5 mg twice daily plus aspirin 75 to 100 mg once daily for a median of 374 days. They were followed every 6 months to evaluate adherence and safety, and to collect clinical outcomes, including stroke, MI, and mortality.
The COMPASS study was a collaboration between Bayer, Janssen Research & Development, LLC and the Population Health Research Institute (PHRI), a joint institute of McMaster University and Hamilton Health Sciences.
WHAT IS XARELTO (rivaroxaban)?
XARELTO is a prescription medicine used to:
• reduce the risk of stroke and blood clots in adults who have a medical condition called atrial fibrillation that is not caused by a heart valve problem. With atrial fibrillation, part of the heart does not beat the way it should. This can lead to the formation of blood clots, which can travel to the brain, causing a stroke, or to other parts of the body
• treat blood clots in the veins of your legs (deep vein thrombosis or DVT) or lungs (pulmonary embolism or PE)
• reduce the risk of blood clots happening again in adults who continue to be at risk for DVT or PE after receiving treatment for blood clots for at least 6 months
• help prevent a blood clot in the legs and lungs of adults who have just had hip or knee replacement surgery
• help prevent blood clots in certain adults hospitalized for an acute illness and after discharge, who are at risk of getting blood clots because of the loss of or decreased ability to move around (mobility) and other risks for getting blood clots, and who do not have a high risk of bleeding
XARELTO is used with low dose aspirin to:
• reduce the risk of serious heart problems, heart attack and stroke in adults with coronary artery disease (a condition where the blood supply to the heart is reduced or blocked)
• reduce the risk of a sudden decrease in blood flow to the legs, major amputation, serious heart problems or stroke in adults with peripheral artery disease (a condition where the blood flow to the legs is reduced) and includes adults who have recently had a procedure to improve blood flow to the legs
XARELTO is used in children to:
• treat blood clots or reduce the risk of blood clots from happening again in children from birth to less than 18 years, after receiving at least 5 days of initial treatment with injectable or intravenous medicines used to treat blood clots.
• help prevent blood clots in children 2 years and older with congenital heart disease after the Fontan procedure.
XARELTO was not studied and is not recommended in children less than 6 months of age who:
• were less than 37 weeks of growth (gestation) at birth • had less than 10 days of oral feeding, or
• had a body weight of less than 5.7 pounds (2.6 kg)
For more information: www.janssen.com
References:
1 Verma, S, Eikelboom, J, Al-Omran, M, et. al. Life and Limb Protection with Dual Anti-thrombotic Pathway Inhibition: COMPASS Ushers in a New Day in Atherothrombotic Risk Reduction. Med. 2020; 2(3): P233-242. doi: https://doi.org/10.1016/j.medj.2020.05.003
2 Fox, K, Aboyans, V, Debus, ES, et al. Patients selected for dual pathway inhibition in clinical practice have similar characteristics and outcomes to those included in the COMPASS randomized trial: The XATOA Registry. European Heart Journal - Cardiovascular Pharmacotherapy. 2022 [Insert doi once live]
3 World Health Organization. Cardiovascular diseases (CVDs). Accessed March 24, 2022 from https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
4 Roth, G., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. Journal of the American College of Cardiology. 2020; 76(25): 2982-3021.
5 Centers for Disease Control and Prevention. Heart Disease in the United States. Accessed March 24, 2022 from https://www.cdc.gov/heartdisease/facts.htm.
6 National Heart, Lung, and Blood Institute. Peripheral Artery Disease. Accessed March 24, 2022, from https://www.nhlbi.nih.gov/health-topics/peripheral-artery-disease
7 Afzal N, Sohn S, Scott CG, Liu H, Kullo IJ, Arruda-Olson AM. Surveillance of peripheral arterial disease cases using natural language processing of clinical notes. AMIA Jt Summits Transl Sci Proc. 2017;2017:28-36. Retrieved June 2, 2021 from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5543345/#r2-2609862.
8 Racial Disparities in Vascular Care. (n.d.). Accessed March 24, 2022 from https://cardiovascularcoalition.com/ourpatients/racial-disparities-in-vascular-care/
9 American Heart Association. PAD Toolkit for Health Care Professionals. Accessed March 16, 2022, from https://www.heart.org/en/health-topics/peripheral-artery-disease/pad-toolkit
* John Eikelboom is the lead author of the COMPASS trial and LTOLE study. He is affiliated with the Population Health Research Institute, Hamilton Health Sciences and McMaster University. The Population Health Research Institute was provided a grant for their participation in both studies.
** Keith Fox is the lead author of the XATOA registry and has received compensation.


 July 31, 2024
July 31, 2024 









