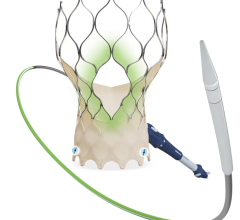
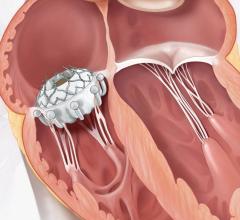
September 20, 2023 — The Minneapolis Heart Institute Foundation (MHIF), an internationally renowned cardiovascular research organization, announced the launch of a clinical trial to evaluate transcatheter aortic valve replacement using the Edwards SAPIEN valve platform in patients with moderate, calcific aortic stenosis. MHIF is working with physician research partners to enroll in this study at United Hospital in St. Paul.
Calcific aortic stenosis (AS) occurs when calcium builds up inside the aortic valve and causes the opening of the valve to become smaller, preventing it from opening and shutting properly. Patients with moderate, calcific AS may or may not experience fatigue, shortness of breath, chest pain, and rapid heartbeat.
The current treatment for patients with moderate AS is careful observation, or clinical surveillance. Clinical surveillance involves prescribing a regimen of medications accompanied by physician visits every 1-2 years. Valve replacement is currently not recommended until aortic stenosis progresses to a severe state. However, some patients with moderate aortic stenosis and added risk factors may or may not benefit from having their aortic valve replaced.
The PROGRESS study, led by Bilal Murad, MD, cardiologist and researcher with MHIF at United Hospital in St. Paul and principal investigator for the study, is examining the transcatheter aortic valve replacement (TAVR) procedure versus careful clinical surveillance in patients with moderate, calcific aortic stenosis. TAVR is a minimally invasive procedure that uses a catheter to implant a new valve within the diseased valve. The TAVR procedure is most commonly performed through a small incision in the groin.
“We are pleased to participate in the PROGRESS trial to contribute to understanding the potential for TAVR as an option for moderate AS,” said Dr. Murad. “There is growing evidence that moderate aortic stenosis may not be as benign as once thought to be and patients struggle with symptoms and impaired quality of life. The goal of this study is to discover if earlier interventions can lead to health improvements in patients by stopping or preventing the worsening of damage to the heart.”
“The United Heart Team functions as a multidisciplinary team across cardiology, cardiac surgery, imaging, and with robust nursing and advanced provider support to help provide patients with unique treatment options not available everywhere,” said Nishtha Sodhi, MD, cardiologist and researcher with MHIF, and director of the Allina Health Minneapolis Heart Institute Structural Heart and Valve Program at United Hospital. “We are grateful to have been a site chosen to participate in this important study.”
Patients who qualify for the PROGRESS trial will be randomized between TAVR with the Edwards SAPIEN valve platform and clinical surveillance. This means that patients have a 50/50 chance of being in the TAVR group or the clinical surveillance group. If you are assigned to the clinical surveillance group and your aortic stenosis worsens, your doctor will determine the best treatment option for you – which can include valve replacement.
The purpose of the trial is to compare results of patients who have their valves replaced at a moderate level of disease versus patients that have their disease monitored.
Patients interested in participating in the study should contact their doctor to learn more or call 651-241-2730. The United Heart Team is comprised of Bilal Murad, MD; James Kolbeck, MD; Brian Mahoney, MD; Nishtha Sodhi, MD; Gabriel Rodriguez Olivares, MD; Robert Steffen, MD; Carrie Hiemstra, RN; Jessica Timmerman, RN; Danielle Mitchell; Mary Biagini, MSN, RN; and Kelly Jo Powerstorm, MHA-MBA.
For additional details on the study, visit thePROGRESStrial.com or go to clinicaltrials.gov
CAUTION: INVESTIGATIONAL DEVICES. The Edwards SAPIEN 3 and Edwards SAPIEN 3 Ultra transcatheter heart valves are investigational devices when used in patients with moderate aortic stenosis. Limited by Federal (USA) law to investigational use only. These devices are not available for marketing or commercial sale in the United States for patients with moderate aortic stenosis.
For more information: www.mplsheart.org
Related content:
Edwards Launches Sapien 3 Ultra Resilia Valve Following FDA Approval
Edwards Sapien 3 TAVR Valve Receives Expanded Approval in Canada


 July 08, 2024
July 08, 2024