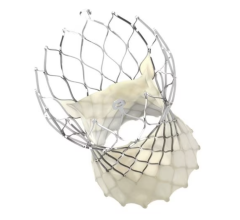
May 11, 2018 — Medtronic plc announced two-year outcomes for the Harmony Transcatheter Pulmonary Valve (TPV) from its early feasibility study at the Society for Cardiovascular Angiography and Interventions (SCAI) 41st Annual Scientific Sessions, April 25-28 in San Diego. Data from 18 patients followed out to two years revealed the Harmony TPV showed solid valve function and no paravalvular leak (PVL).
“Following the one-year feasibility outcomes, we are encouraged to see the Harmony valve continues to show positive outcomes for patients two years post-implant,” said Matthew J. Gillespie, M.D., cardiologist at The Cardiac Center at Children's Hospital of Philadelphia, who presented the data at the meeting. “We are optimistic that these early outcomes will be a strong indicator of the types of results that we might expect to see from our pivotal study, which is currently enrolling.”
Designed to offer a treatment alternative for patients with congenital heart disease (CHD), the Harmony TPV is being studied in CHD patients born with right ventricular outflow tract (RVOT) anomalies who undergo a surgical repair early in life. For these patients, who account for approximately 80 percent of CHD patients born with RVOT anomalies, the Harmony TPV provides a less invasive option to help restore normal valve function later in life.
Two-year results were consistent with one-year outcomes presented at the 2016 Transcatheter Cardiovascular Therapeutics annual meeting (TCT16). Patients enrolled in the Harmony TPV early feasibility study who have now been followed out to two years (N=18) continued to experience strong hemodynamics (blood flow), with 86.7 percent of patients having no/trace pulmonary regurgitation (PR) at two years. Mean gradients were consistent and stable at two years follow-up and there were no paravalvular leaks reported. Two patients experienced tissue growth within the stent frame and were treated successfully with a transcatheter valve-in-valve procedure with the Melody TPV.
The Harmony TPV is available for investigational use only. The Harmony Pivotal IDE Study is treating up to 40 patients at approximately 15 sites in the U.S., Canada and Japan.
Complete listing of SCAI 2018 late-breaking trials with links to articles.
For more information: www.medtronic.com


 May 05, 2025
May 05, 2025