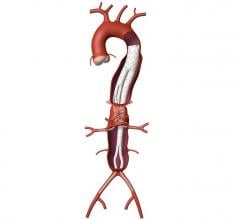
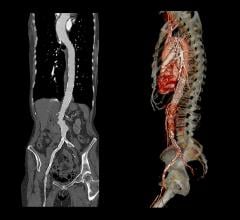
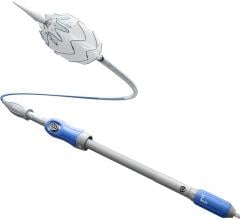
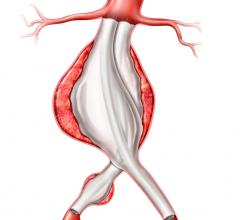
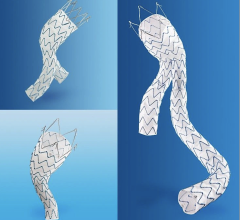
June 5, 2008 - The FDA has just approved Medtronic’s Talent Thoracic Stent Graft for endovascular treatment for descending aneurysms and penetrating ulcers of the thoracic aorta. The approval gives endovascular interventionalists in the United States access to an innovative medical device that will bring treatment to an additional 25 percent of patients.
The Talent Thoracic Stent Graft System is available in a wide range of diameters from 22 mm to 46 mm. This size matrix offers physicians multiple options to customize devices for their patients needs and to treat a wider range of anatomies than with the other available endografts.
For more information: www.medtronic.com

 April 26, 2023
April 26, 2023