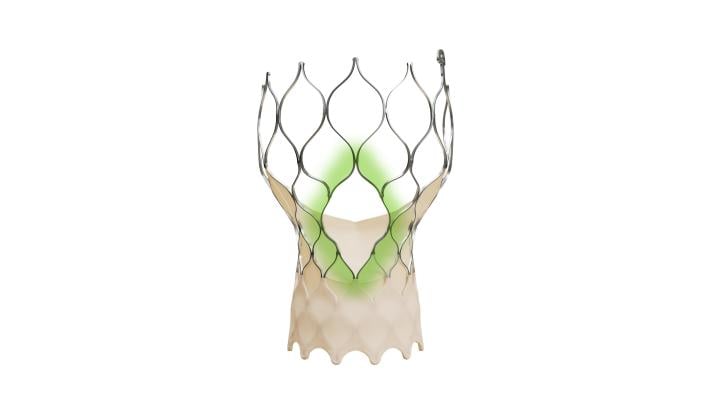
March 28, 2024 — Medtronic plc, a global leader in healthcare technology, announced that the United States Food and Drug Administration (FDA) has approved the Evolut FX+ transcatheter aortic valve replacement (TAVR) system for the treatment of symptomatic severe aortic stenosis. The latest Evolut FX+ TAVR system maintains the valve performance benefits of the legacy Evolut TAVR platform and is designed to facilitate coronary access.
The Evolut FX+ TAVR system offers larger coronary access windows through a modified diamond-shaped frame design, which is four times larger than previous iterations of the Evolut TAVR system. Evolut FX+ provides increased space for catheter maneuverability to facilitate access to coronary arteries of varying patient anatomies. Additionally, the new design does not compromise the market leading valve performance, excellent hemodynamics, and radial strength that clinicians expect from the Evolut platform.1
"We are committed to consistently developing and advancing minimally invasive solutions for physicians to treat their patients with aortic stenosis. This is reinforced by our continued innovation of the Evolut TAVR platform, which has delivered proven valve performance and durability to physicians and patients for years. The Evolut FX+ TAVR system was designed to facilitate coronary access across a diverse range of patient anatomies with no compromise to valve performance," said Jeffrey Popma, M.D., vice president and chief medical officer for the Coronary & Renal Denervation business and the Structural Heart & Aortic business, which are part of the Cardiovascular Portfolio at Medtronic.
Severe aortic stenosis occurs when the aortic valve leaflets become stiff and thickened and have difficulty opening and closing, making the heart work harder to pump blood to the rest of the body. Severe aortic stenosis often reduces a patient's quality of life and limits their daily activities. If left untreated, 50% of patients with symptomatic severe aortic stenosis can die from heart failure in as little as two years.2
The Evolut FX+ TAVR system is indicated for symptomatic severe aortic stenosis patients across all risk categories (extreme, high, intermediate, and low) in the U.S. Early Commercial Experience is planned for spring 2024 with full product launch anticipated in summer 2024.
For more information: www.medtronic.com
Fine more ACC24 conference coverage here
References:
1Medtronic data on file compared to the Evolut platform. Bench top model may not be indicative of clinical performance.
2Ross J Jr, Braunwald E. Aortic stenosis. Circulation. July 1968; 38(1 Suppl):61-67.


 July 31, 2024
July 31, 2024 









