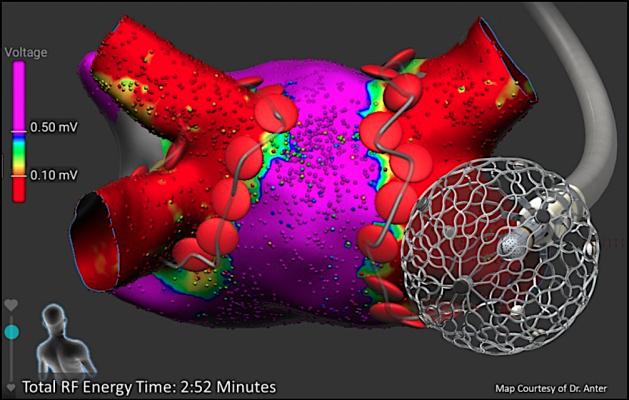
The Affera Inc. Sphere-9 pulsed field (PF) energy radiofrequency ablation system. Medtronic purchased the company in January 2022.
January 14, 2022 — Medtronic has entered into a definitive agreement to acquire Affera Inc., a Boston area-based, privately held medical technology company. Affera designs and manufactures electrophysiology (EP) cardiac mapping and navigation systems and catheter-based cardiac ablation technologies. This expands Medtronic's entry into the mapping and ablation navigation system market and flushes out its EP offers.
One of the key technologies Affera has develop a focal pulsed field ablation solution to treatment of patients with cardiac arrhythmias. Pulsed field ablation, also known as electroporation because it causes cell death by causing pores to open in cell the cell walls of the target tissue, is expected to be an advancement in EP because it hold promise for causing less collateral damage to surrounding healthy tissues than conventional ablation methods. Medtronic, through its minority investment portfolio, has been a strategic investor in Affera and currently holds a 3% ownership stake in the company.
Medtronic said the acquisition expands the Medtronic portfolio of advanced cardiac ablation products and accessories to meet physician needs within a growing patient population. Affera's technologies include the Affera Prism-1 cardiac mapping and navigation platform and Sphere-9 cardiac ablation catheter, investigational technologies designed to enable the rapid creation of detailed maps used by electrophysiologists to diagnose arrhythmias and ablation cardiac tissue causing arrhythmias. The Affera suite of solutions and technologies will complement the existing Medtronic atrial and ventricular arrhythmia disease management portfolio and support the company's efforts to offer simple, safe and effective cardiac ablation solutions to improve patient outcomes, Medtronic said.
"The EP ablation market is an exciting and fast-moving segment of cardiology," said Rebecca Seidel, president of the cardiac ablation solutions (CAS) business, which is part of the cardiovascular portfolio at Medtronic. "Bringing Affera into our organization, with our established footprint in the cardiac ablation space, will strengthen our ability to provide innovative therapies and enable Medtronic entry into additional EP technology segments, such as mapping and navigation, for the first time."
"Affera offers technologies that support physician customers as they work to improve clinical workflows, procedural efficiencies, and ultimately optimize patient care," said Stacy Beske, Ph.D., vice president of strategy, CAS.
Within the $8 billion worldwide EP ablation market, the prevalence of cardiac arrhythmias is growing rapidly, and the need to provide treatment to the increasing patient population, which encompasses AF, supraventricular tachycardia (SVT), and ventricular tachycardia (VT), is increasing. Atrial fibrillation (AF) represents the largest disease segment, with nearly 60 million people affected worldwide.[1] AF is a progressive disease, meaning over time patients can experience more frequent, and longer episodes, and medication as well as catheter ablation can become less effective. Additionally, AF is associated with serious complications including heart failure, stroke, and increased risk of death.[2-5]
"This is an exciting day for patients who suffer from the burden of AF and other arrhythmias. This acquisition directly aligns with our vision of delivering novel solutions to address the rapidly growing demands for cardiac arrhythmia treatment," said Doron Harlev, founder and chief executive officer of Affera. "We are excited to focus on the integration of our technology with Medtronic and are confident that together we can increase patient access to ablation therapies."
In December 2021, Affera announced the commencement of the recently approved SPHERE PerAF Trial, a U.S. Food and Drug Administration (FDA) investigational device exemption (IDE) pivotal randomized trial, to evaluate the safety and effectiveness of the Affera system for the treatment of persistent AF. Affera's product portfolio is not currently approved or available for sale or commercial use.
Financial Highlights of Medtronic's Purchase of Affera
The acquisition is expected to close the first half of Medtronic fiscal year 2023, subject to the satisfaction to certain customary closing conditions. Following close, the transaction is expected to be less than 1% dilutive to Medtronic's adjusted earnings per share in each of the first three years, and neutral to accretive thereafter. The company expects dilution of approximately 5 cents in both year 1 and year 2 and approximately 3 cents in year 3.
In collaboration with leading clinicians, researchers, and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services of the highest quality that deliver clinical and economic value to healthcare consumers and providers around the world.
For more information: www.affera.com, www.Medtronic.com
Related Pulsed Field Ablation Technology:
Affera Pulsed Field Ablation Successfully Treats Atrial Fibrillation
Medtronic FDA Trial Evaluates Pulsed Electric Fields to Treat Atrial Fibrillation
Pulsed AF Trial Shows Pulsed Field Ablation May be Safer Than Tranditional RF Ablations.
Boston Scientific Invests in Pulsed Field Ablation Technology To Improve Atrial Fibrillation Therapy
First Patients Treated with Galaxy Medical Centauri Pulsed Electric Field Cardiac Ablation System
References:


 January 29, 2026
January 29, 2026 









