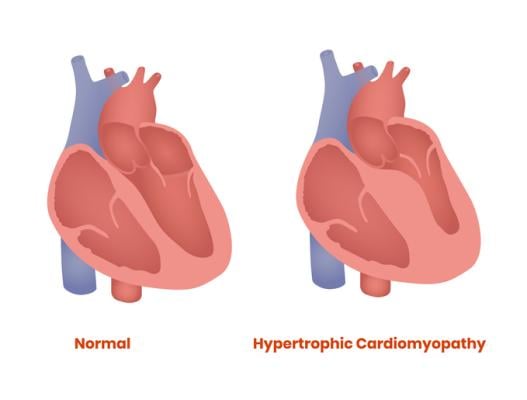
April 12, 2022 – A majority of patients with obstructive hypertrophic cardiomyopathy (HCM)—a condition that results in excess thickening of the heart muscle—who were candidates for a procedure to relieve the obstruction of blood flow out of the heart no longer needed the surgery after taking the investigational drug mavacamten for 16 weeks, in a phase 3 study presented at the American College of Cardiology’s 71st Annual Scientific Session.
Only 17.9% of patients on mavacamten were still eligible for septal reduction therapy—a procedure to relieve the obstruction of blood flow to the heart—at the end of the study period, compared with 76.8% of those on placebo. Researchers also observed significant improvements in symptoms, quality of life, degree of obstruction to blood flow and key blood markers for heart damage and heart failure in patients taking mavacamten.
“These were very symptomatic, sick patients who were on maximally tolerated medical therapy and were faced with the decision of whether or not to have septal reduction therapy to relieve the obstruction. In taking this drug, they got a whole lot better across the board,” said Milind Y. Desai, MD, MBA, director of the Hypertrophic Cardiomyopathy Center, director of clinical operations in Cleveland Clinic’s Heart Vascular and Thoracic Institute and the study’s lead author. “This is really the first pharmacotherapy that offers a viable medical option for people with obstructive HCM short of needing a procedure.”
HCM is the most common genetic heart condition. It’s estimated to affect 1 in 500 adults, or roughly 20 million people worldwide, yet most people don’t know they have it. With HCM, changes in certain genes allow the heart muscle to get too thick (“hypertrophy” means to thicken). In some cases, the left ventricular outflow tract (LVOT) where blood leaves the heart becomes obstructed by the thickened heart muscle, forcing the heart to work harder and leaving people unable to do even simple tasks without feeling winded and fatigued. Obstructive HCM is also associated with increased risks of atrial fibrillation, stroke, heart failure and, although rare, sudden cardiac death.
Treatment for severe obstructive HCM symptoms has been septal reduction therapy either through surgical myectomy by opening the chest and shaving a piece of the muscle or by injecting alcohol in one of the coronary arteries to shrink the heart muscle and relieve the obstruction. VALOR-HCM was a randomized, double-blind, placebo-controlled study that evaluated the use of mavacamten in adults with symptomatic obstructive HCM who were referred to an HCM expert to consider having one of these therapies. A total of 112 patients were enrolled at 19 HCM centers. Patients were randomly assigned to receive mavacamten or a placebo for 16 weeks to see if taking the medicine could delay or eliminate the need for surgery.
“These are pretty aggressive therapies,” Desai said. “They work well when done in a highly experienced HCM center where mortality is low, less than 1%, but most centers don’t do enough, and the mortality rate can be high.”
In fact, outside of highly experienced centers, one analysis reported the average rate of postoperative death to be 5.9%, ranging from 3.8% in higher volume centers to 15.6% in lower volume centers. Septal reduction therapies usually come after medications fail; clinicians will often use beta blockers, some types of calcium channel blockers and disopyramide, but to date there have been limited studies evaluating their clinical benefit for HCM, researchers said.
“There is clearly an unmet need to develop targeted therapies for this disease, especially when we consider lack of broad availability of specialized care,” Desai said.
Patients receiving mavacamten started on a 5 mg dose, which was adjusted based on echocardiogram measures of ejection fraction (the heart’s squeezing ability) and the LVOT gradient. Doses ultimately ranged from 2.5 mg to 15 mg. Mavacamten was added on to maximally tolerated medical therapy. Patients were evaluated by a clinical exam and echocardiogram at baseline, once monthly and at 16 weeks. The primary endpoint was how many patients would still meet eligibility for, or choose to receive, septal reduction therapy. At 16 weeks, 43 out of 56 (76.8%) assigned to placebo vs. 10 out of 56 (17.9%) of those receiving mavacamten were still found to meet guideline criteria for septal reduction therapy (defined as LVOT gradient of ≥50 mmHg and NYHA Class III-IV)—a striking and significant difference. At baseline, 93% of patients were NYHA Class III or higher with severe obstruction.
Researchers also collected data on multiple secondary outcome measures, including changes in post-exercise LVOT; the proportion of patients whose NYHA Class, an indicator of disease severity, improved; changes in the Kansas City Cardiomyopathy Questionnaire (KCCQ), which assesses patients’ report of symptoms and physical limitations; as well as NT-proBNP and troponin levels. At 16 weeks, all were found to be significantly improved among patients taking mavacamten compared with those on placebo. Researchers highlighted significant improvement in NYHA class among patients taking mavacamten, with 63% patients improving by one and 27% improving by two NYHA classes.
Mavacamten is a selective cardiac myosin inhibitor and has been shown to reduce cardiac muscle contractility by inhibiting excessive myosin-actin cross-bridge formation that results in increased contraction, excessive thickening of the heart muscle and reduced compliance (increased stiffness). Because it reduces hypercontractility, there was some concern that it might affect heart function. Desai said that the drug was well tolerated. Two patients in the mavacamten group had a drop in ejection fraction to less than 50% and had to stop the drug temporarily until their ejection fraction recovered, but they were able to restart the drug at a lower dose. There were no reported deaths, strokes or heart attacks.
Patients were offered the opportunity to enroll in the extension study for three years to receive mavacamten or undergo a procedure, and nearly all of them (95%) chose to take the drug. This will allow the research team to see how many patients end up crossing over and needing surgery, and how many can stay on the medication.
“This is a big win for patients, especially as many people don’t want surgery, are at high risk for complications or don’t have good anatomy for ablation,” Desai said. “We are hoping this study will also draw much-needed attention to HCM. This disease is woefully underrecognized. There are not enough HCM experts and not enough proven medical therapies, so there are many opportunities to do better.”
Mavacamten is currently under review with the U.S. Food and Drug Administration for use in obstructive HCM, with a decision expected at the end of April. The study was funded by MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb.


 July 31, 2024
July 31, 2024 









