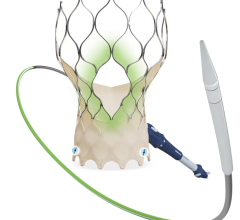
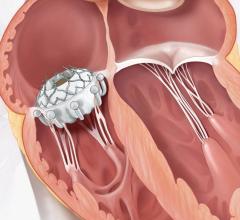
August 19, 2015 — Loyola University Medical Center is the first center in Illinois to implant the CoreValve Evolut R percutaneous aortic heart valve that does not require open heart surgery.
The device is deployed with a catheter, which is inserted into an artery and guided to the heart. Once in place, the artificial valve takes over the function of the diseased valve. The system is much less invasive than open surgery.
The newly designed system enables the physician to recapture and reposition the device in order to obtain a more accurate deployment every time. Recently approved by the U.S. Food and Drug Administration (FDA), it is the first and only recapturable and repositionable device commercially available in the United States. It sets a new standard for transcatheter aortic valve replacement (TAVR) in the United States.
J. Michael Tuchek, DO, lead implanter of the CoreValve device at Loyola, and Bruce Lewis, M.D., implanted the first CoreValve Evolut R System in Illinois. Tuchek, who consulted in the engineering and design of the device, is a clinical associate professor in the Department of Thoracic and Cardiovascular Surgery. Lewis is an interventional cardiologist and a professor in the Department of Medicine.
Tuchek said the unique design of the CoreValve Evolut R System “allows for superior control during deployment of the CoreValve, along with the ability to recapture and reposition the valve.”
Tuchek noted the device is the smallest-diameter TAVR device that is commercially available. “The new device also optimizes the valve design to enhance sealing and thus prevent aortic insufficiency postoperatively,” he said.
The system represents a significant advance in the TAVR field, said Fred Leya, M.D., Loyola’s director of interventional cardiology. “There’s no question that this is a superior product,” he said. “The technology is a game changer for patients with aortic stenosis.” Leya is a professor in the Division of Cardiology of Loyola University Chicago Stritch School of Medicine.
Loyola was the only center in Illinois to participate in a landmark clinical trial of CoreValve, which was published in the New England Journal of Medicine. The study found that patients who received the device had significantly lower mortality than heart valve patients who underwent open-heart surgery.
Loyola has now been implanting CoreValves for more than four years. The implanting team includes two cardiovascular surgeons (Tuchek and Mamdouh Bakhos, M.D.) and two interventional cardiologists (Leya and Lewis). The team is participating in ongoing CoreValve trials and leads the state in implanting the device.
The improved device now is FDA-approved for patients judged to be at high or extremely high risk for conventional open-heart aortic valve surgery (with an estimated 30-day mortality rate of at least 15 percent). Loyola is the only site in Illinois participating in the SURTAVI (SURgical vs. Transcatheter Aortic Valve Implantation) clinical trial, in which the device is being implanted in lower-risk patients. It is being conducted in 76 sites in eight countries for patients who do not qualify for the transcatheter valves commercially. The trial provides the only opportunity for lower-risk patients (called intermediate risk) to receive CoreValve.
In addition, extreme-risk patients with other valve disorders are being enrolled in the Expanded Use Study (EUS). Leya and Bakhos are principal investigators at the Loyola site for the SURTAVI and EUS trials.
For more information: www.loyolamedicine.org


 July 08, 2024
July 08, 2024