March 29, 2012 — Non-cardiac co-morbidities such as chronic obstructive pulmonary disease, chronic kidney disease and frailty are the main predictors of late mortality after transcatheter aortic valve implantation (TAVI), suggesting that patients with these conditions merit closer evaluation and follow-up. This was according to research presented this week at the American College of Cardiology’s (ACC) 61st Annual Scientific Session.
The study provides the longest multicenter follow-up data on the clinical outcomes and valve durability of TAVI, a procedure that has been developed to repair or replace a diseased heart valve in patients who cannot undergo surgery. The minimally invasive procedure consists of inserting a bioprosthetic valve through a catheter into the heart to take the place of the diseased valve.
“While TAVI has emerged as the treatment of choice in non-operable patients who need valve repair, most data on TAVI are limited to acute and one-year follow-up,” said Josep Rodés-Cabau, M.D., lead study author and director of the cardiac catheterization and interventional laboratories at the Quebec Heart and Lung Institute. “Also, very few data exist on the long-term durability of transcatheter valves. Identifying the clinical factors associated with poorer late outcomes is very important in improving patient selection and follow-up, while properly evaluating valve durability is one of the most important factors determining the potential expansion of TAVI to younger and lower-risk patients.”
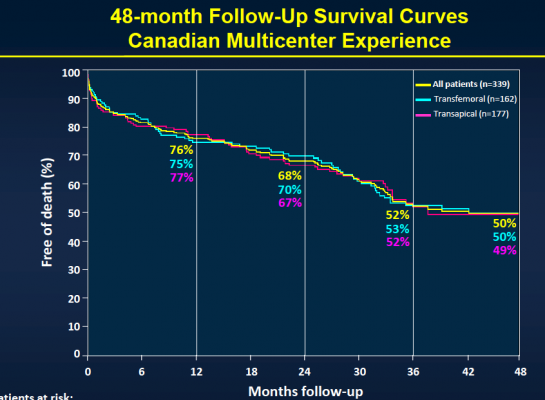
The study included 339 patients considered to be non-operable or at very high surgical risk who underwent TAVI at six Canadian medical centers between January 2005 and June 2009. The transfemoral approach was used in 48 percent of the patients, while the transapical approach was used in 52 percent of the patients. Patients received a balloon-expandable valve (Cribier-Edwards, n=57), (Edwards Sapien, n=275), (Sapien XT, n=7) and were then followed to determine both safety and efficacy. Clinical follow-up to determine safety was conducted in clinical visits and/or through phone contact, while efficacy follow-up was performed through echocardiography with central echo core lab evaluation.
After the median three-year follow-up, 146 (43.1 percent) of the patients had died. Thirty-six (10.4 percent) of those patients died within 30 days of TAVI, and 110 patients (32.4 percent) died during the remainder of the follow-up period. Of those who died more than 30 days post-procedure, 74 (67.3 percent) died because of non-cardiac causes, including pulmonary causes (32.7 percent) and end stage kidney disease (10 percent). Twenty-nine patients (26.4 percent) died of cardiac causes. There were no cases of valve structural failure, and there were two cases of re-intervention because of valve endocarditis, infection of the valves.
For more information: www.acc.org






 January 05, 2026
January 05, 2026 









