May 31, 2024 — CareDx, a leading precision medicine company focused on the discovery, development, and commercialization of clinically differentiated, high-value healthcare solutions for transplant patients and caregivers, announced findings from the SHORE (Surveillance HeartCare Outcomes Registry) study, one of the largest heart transplant studies of its kind, published in The Journal of Heart and Lung Transplantation.
The prospective observational study demonstrates that HeartCare which combines AlloSure Heart donor-derived cell-free DNA (dd-cfDNA) and AlloMap Heart gene-expression profiling (GEP), identifies acute cellular rejection in heart transplant patients better than dd-cfDNA testing alone and is associated with fewer biopsies and excellent clinical outcomes.
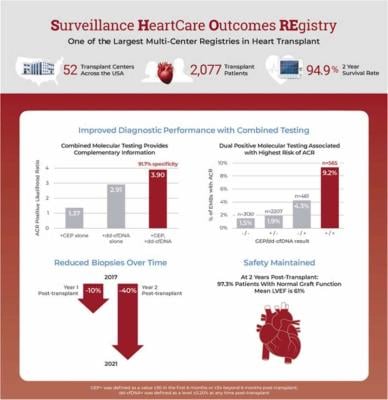
Key Findings from the SHORE Study Publication
- Dual-positive HeartCare results are associated with the highest incidence (9.2%) of acute cellular rejection (ACR), whereas dual-negative HeartCare results are associated with the lowest incidence (1.5%) of ACR.
- Follow-up endomyocardial biopsies (EMB) are performed at the lowest rate (8.8%) following a dual-negative HeartCare result, and at the highest rate (35.4%) following a dual positive HeartCare result.
- The rate of biopsies performed in response to a single positive result is 56% lower than dual-positive HeartCare results.
- Clinician behavior to HeartCare results changed over time as centers gained experience with the testing. By the end of the study cardiologists performed 10% fewer biopsies in the first year post-transplant and 40% fewer in the second year post-transplant despite an increase in follow-up biopsy rates for dual positives.
- Excellent clinical outcomes were observed in patients managed with HeartCare including fewer follow-up endomyocardial biopsies, 95% survival and 97% with normal allograft function at two years post-transplantation.
“This large, prospective, multicenter study demonstrates that HeartCare significantly improves clinicians’ ability to assess acute cellular rejection risk and its use is associated with lower biopsy rates and excellent clinical outcomes two-years post-transplant,” said John W. Hanna, CareDx President and CEO.
“The SHORE study confirms the superior performance of dual molecular testing, combining donor-derived cell-free DNA and gene-expression profiling for acute cellular rejection surveillance in optimizing care for heart transplant patients. This approach allows for refined patient selection for surveillance biopsies, leading to fewer invasive procedures over time while maintaining vigilant ACR monitoring, and achieving excellent clinical outcomes,” said Kiran Khush, MD, MAS, Cardiologist, Professor of Cardiovascular Medicine, Stanford Medicine.
One of the largest heart transplant studies of its kind, SHORE is a prospective 67-center, observational study of over 2,700 heart transplant patients in the United States receiving non-invasive molecular testing with AlloSure Heart dd-cfDNA and AlloMap Heart GEP or HeartCare. Together, these different molecular tests offer a more comprehensive evaluation of a patient’s heart transplant status by assessing both allograft health and immune system activity. The published study was designed to evaluate the utility of combined molecular testing using HeartCare for acute cellular rejection (ACR) surveillance.
For more information: https://caredx.com/


 May 30, 2025
May 30, 2025 









