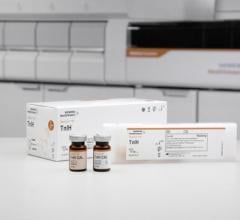
November 18, 2015 — Konica Minolta Inc. announced that it has developed the SPFS Immunoassay System, capable of highly sensitive measurements based on the fluorescent antibody technique. A prototype will be on reference exhibit at MEDICA 2015, the world’s largest international medical equipment fair in Dusseldorf, Germany, Nov. 16-19, 2015.
As a diagnostic method for myocardial infarction, malignancies and other pathologic conditions, the fluorescent antibody technique is used to detect disease-associated biomarkers (proteins) in the blood by means of the light generated with the use of a labeled antibody (an antibody coupled with a chemical substance known as a fluorescent marker). Recognizing that its technologies could be useful for increasing the sensitivity of the fluorescent antibody technique, Konica Minolta has been developing the SPFS Immunoassay System.
Specifically, the company’s nanotechnology and fine chemical technology that have emerged and evolved from the development of photographic films make chemical reactions highly controllable, and allow target antibodies to be detected with high sensitivity at the molecular level. In addition, the lens design know-how and light-guiding technology honed through the development of cameras are expected to be particularly useful in the detection of weak light and fluorescence, as well as other situations, thus improving the performance of such tests.
Among acute myocardial infarction patients who are admitted to hospital for emergency treatment for chest pain, those with non-ST elevation acute coronary syndromes are expected to enjoy shorter recovery times after treatment and lower mortality rates by starting early diagnosis and treatment with the use of highly sensitive testing. However, the conventional test with troponin (a biomarker (protein) in heart muscle), which is currently used for diagnosing myocardial infarction, requires about 6 hours for diagnosis, and is reported to have accuracy problems, causing various major issues in emergency medicine, including overcrowding.
Konica Minolta has developed the SPFS Immunoassay System, which comprises a compact unit for highly sensitive measurement of troponin with a diagnosis time of just 3 hours, and a set of heart disease-related reagents, including troponin. The system will be able to dramatically increase the efficiency of diagnostic and other clinical activities, and lessen overcrowding in emergency care settings, thus helping to reduce patient mortality rates and facilitate early recovery and discharge.
Furthermore, the SPFS Immunoassay System can accurately detect disease-specific biomarkers (proteins found specifically in target diseases) to provide a highly reliable immunoassay system not only for heart diseases such as myocardial infarction, but also for malignancies and other diseases, thus improving medical care.
Konica Minolta is planning to launch the SPFS Immunoassay System incorporating markers related to heart diseases primarily in Europe in 2017, and then sell it in both the European and American markets.
The company is also planning to create a more efficient immunoassay system with a greater number of test parameters, and to fully enter the in vitro diagnostics market with the high-sensitivity immunoassay system.
For more information: www.konicaminolta.com

 October 09, 2019
October 09, 2019