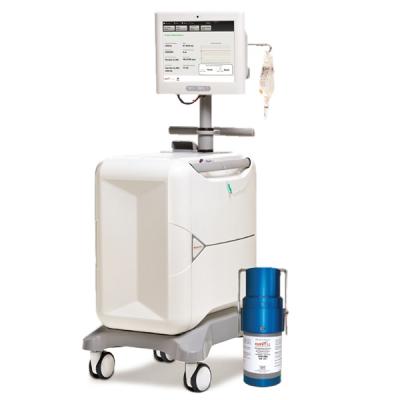
June 26, 2023 — Jubilant DraxImage Inc., dba Jubilant Radiopharma, a wholly-owned subsidiary of Jubilant Pharma Limited, announced the clearance by the FDA to use its RUBY Rubidium Elution System and RUBY-FILL (Rubidium Rb82 generator) in mobile settings. RUBY-FILL is used for cardiac PET imaging under rest or pharmacologic stress conditions to evaluate regional myocardial perfusion in patients with suspected or existing coronary artery disease.
Since the FDA approval of RUBY-FILL in 2016, Jubilant Radiopharma has partnered with health care providers across the U.S. to establish and grow cardiac PET programs. Typically, cardiac PET is performed in larger hospitals, outpatient programs and physician offices whose patient volumes can support a full time cardiac PET program. The announcement of the FDA approval for mobile use with RUBY-FILL will allow Jubilant to expand the use of cardiac PET into smaller community hospitals, in rural settings, and in areas where a full time cardiac PET program may not be warranted.
“The approval for mobile use with RUBY-FILL for cardiac PET is another example of Jubilant Radiopharma’s ongoing commitment to expand this imaging modality and increase patient access to this crucial test”, said Sergio Calvo, President, Jubilant Radiopharma, Radiopharmaceuticals Division. “Offering our customers the option to start a cardiac PET program on a part-time basis allows them to fully assess the value of initially offering the diagnostic test and making the determination how it will fit into a broader program for their patients in the future”.
Jubilant Radiopharma will have a booth at the Society of Nuclear Medicine held June 24-27, 2023 at the McCormick Convention Center in Chicago, booth 2065. Our representatives will be on hand to answer questions.
For more information: www.RUBY-FILL.com

 June 28, 2023
June 28, 2023 
