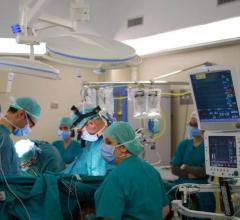
Andrei, 12, is the world’s youngest patient to be bridged to transplant with the SynCardia Total Artificial Heart. He is pictured after his heart transplant with his surgeon, Antonio Amodeo, M.D., at Bambino Gesù Children’s Hospital in Rome, Italy. Photo courtesy of Business Wire.
June 22, 2017 — A 12-year-old boy at Bambino Gesù Children’s Hospital in Rome, Italy, has become the world’s youngest patient to be bridged to a heart transplant with the SynCardia temporary Total Artificial Heart.
Diagnosed with dilated cardiomyopathy, Andrei began experiencing biventricular dysfunction in January 2017. Two months later, he was admitted to the hospital with end-stage heart failure. The 12-year-old needed a heart transplant, but a matching donor heart wasn’t available.
To save Andrei’s life, on April 21, doctors removed his failing heart and implanted the 50cc Total Artificial Heart to restore blood flow to his body and help him get stronger for transplant. Two weeks later, Andrei received the matching donor heart he’d been waiting for.
“I use the Total Artificial Heart because it is more versatile than BIVAD support with the Berlin Heart, the only alternative, and the Total Artificial Heart permits patients to be discharged from the hospital,” said Antonio Amodeo, M.D., surgical director of the Heart Failure Program at Bambino Gesù Children’s Hospital. “The 50cc Total Artificial Heart was the best option based on the age and size of this patient.”
At the time of implant, Andrei was 17 days younger than the previous record-holder, a 12-year-old girl in the United Kingdom who was successfully bridged to transplant with the 50cc Total Artificial Heart in 2016.
The 50cc Total Artificial Heart is a smaller version of SynCardia’s 70cc Total Artificial Heart, the first and only heart replacement device to attain commercial approval in the U.S., Canada and Europem, according to the company. Designed to fit patients of smaller stature, including more women and adolescents, the 50cc Total Artificial Heart has the CE mark for use in Europe and is undergoing a U.S. Food and Drug Administration (FDA)-approved Investigational Device Exemption (IDE) clinical study in the United States.
Similar to a heart transplant, the SynCardia Total Artificial Heart replaces the failing heart and restores blood flow to the body, allowing the patient to begin their recovery from heart failure. Unlike a donor heart, the Total Artificial Heart cannot be rejected and is readily available at SynCardia Certified Centers, providing a new heart without the wait to patients with end-stage biventricular heart failure.
For more information: www.syncardia.com


 March 31, 2025
March 31, 2025