May 18, 2011 – The world’s first system to integrate the functional and anatomical modalities of fractional flow reserve (FFR) and optical coherence tomography (OCT) into one platform will be highlighted by St. Jude Medical at the Paris Course on Revascularization (EuroPCR) 2011.
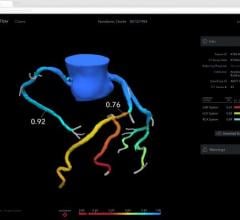
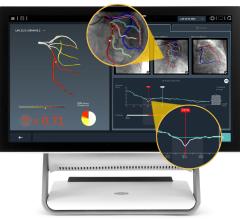
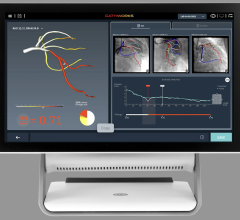
The Ilumien system enables OCT imaging with the third-generation DragonFly OCT catheter, and FFR measurement through wireless connections, which simplify set-up and measurement through the elimination of connecting cables. The combined technology offers percutaneous coronary intervention (PCI) optimization that helps physicians determine if therapeutic intervention is necessary. The Ilumien system is pending European CE mark approval.
St. Jude is also showing its new Wi-Box technology, which enhances connectivity in the cath lab by providing physicians with a quick and easy FFR assessment at any time without any disruptions of the ongoing PCI procedure. The Wi-Box wireless technology enables an easy-to-use, uniform setup solution for all St. Jude Medical FFR platforms including RadiAnalyzer, RadiAnalyzer Xpress and Ilumien.
Late Breaking Trial
Data on potential cost savings in Europe for routine FFR use with the St. Jude Medical PressureWire will be presented by Professor Uwe Siebert, M.D., M.Sc., M.P.H., Sc.D., from the Health and Life Sciences University of Hall (Austria), who led the research project. The Late Breaking Trial is entitled “Cost-effectiveness and public health/budget - Impact of fractional flow reserve-guided PCI in multivessel patients in Europe - Analysis of the FAME study data.”
The analysis, which was funded by an educational research grant from St. Jude Medical, was conducted to determine the incremental cost-effectiveness of FFR-guided vs. angiography-guided PCI in patients with multivessel coronary artery disease in different European health care systems from the societal perspective. Results from France, England, Italy, Switzerland, and Belgium will be revealed on Friday, May 20 at 9:40 a.m. in Room 342AB.
The results of the economic analysis of Germany revealed that an average of nearly 500 deaths and 1,300 heart attacks could be avoided over two years and approximately 14,000,000 Euro could be saved to the German healthcare system with routine FFR use over two years. They were announced this April at Deutsche Gesellschaft fur Kardiologie in Mannheim, Germany.
Products on Display
The company’s products are on display at EuroPCR through May 20 including:
• PressureWire FFR Measurement System: St. Jude Medical pioneered the development of FFR measurements with its PressureWire technology, which indicates the severity of blood flow blockages in the coronary arteries. The PressureWire Aeris and PressureWire Certus provide a physiological measurement that helps physicians to better identify which specific lesion or lesions are responsible for a patient’s ischemia. The benefits of FFR were recognized in the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Guidelines with new recommendations for the treatment of coronary artery disease which support measuring FFR in a wide range of patients before performing PCI or recommending surgery.
• Optical Coherence Tomography (OCT): The C7-XR OCT intravascular imaging system aids physicians in the diagnosis and treatment of coronary artery disease. The OCT coronary imaging technology helps to optimize treatment with stents, and has about 10 times better image resolution and 20 times faster image capture over the current industry standard of Intravascular Ultrasound (IVUS). This efficient technology provides easy visualization, with high resolution images in less than five seconds.
• Amplatzer PFO Occluder: The Amplatzer product line has become the standard of care for patients with structural heart defects. The Amplatzer PFO Occluder technology allows for simple and confident PFO closure using a minimally-invasive transcatheter procedure instead of surgery. The design fits all PFO anatomies and has demonstrated clinical success.
• Amplatzer Cardiac Plug: The Amplatzer Cardiac Plug provides an alternative therapy for reducing the risk of stroke in patients with atrial fibrillation. The unique design of the Amplatzer Cardiac Plug device provides secure placement and self-positioning for optimal occlusion at the orifice of the left atrial appendage.
• Other products: Amplatzer Vascular Plugs, Angio-Seal Vascular Closure Devices, FemoStop II Plus Compression Assist Device, and the RadiStop Compression Assist Device.
For more information: www.sjm.com


 February 03, 2026
February 03, 2026