Impella ECP pump insertion.
Oct. 28, 2024 — Results from the first completed pivotal trial on patients supported with Impella ECP, a novel transvalvular axial flow pump with compressible pump architecture were presented at Transcatheter Cardiovascular Therapeutics (TCT) 2024 conference in Washington, D.C.
Impella ECP is a technology of Abiomed, part of Johnson & Johnson MedTech, and a global leader in heart recovery.
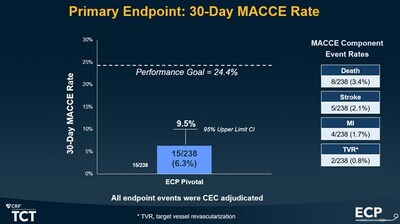
Figure 1. Source: “Mechanical Circulatory Support of High-Risk PCI with a Novel, Low-Profile pMCS: First Results of the Impella ECP Pivotal Study”
The pivotal investigational device exemption (IDE) study, which enrolled 256 patients at 18 sites in the US, met the primary endpoint. MACCE (Major Adverse Cardiac and Cerebrovascular Events) rate at 30 days was 6.3 percent, significantly below the pre-defined performance goal (figure 1). The study demonstrated Impella ECP's safety and efficacy for use in high-risk PCI. Operators chose 8Fr Angio-Seal as the first-closure method in 70 percent of the patients, with a 92 percent success rate1. (See full presentation)
The study was led by Principal Investigator Amir Kaki, MD2, Director of Mechanical Circulatory Support and Complex Coronary Intervention at Henry Ford - St. John Hospital and Medical Center. "The study met its prespecified 30-day primary endpoint with low complication rates and the 9Fr arterial access enabled a high success rate closing with an 8Fr Angio-Seal," said Dr. Kaki. "Impella ECP technology with small-bore access and closure offers benefits for patients and physicians."
With a 9Fr size at insertion, Impella ECP is designed to be implanted and removed using small bore access and closure techniques. After insertion, Impella ECP expands to 21Fr to provide circulatory support and left ventricular (LV) unloading for high-risk PCI.
Impella ECP is an investigational device limited by federal law to investigational use only. Impella ECP is in use as part of the FDA-approved continuous access program at selected US sites. As a next step, Impella ECP will be submitted to the US FDA for approval.
1 Success defined as hemostasis with first and only device and no additional closure strategy.
2 Dr. Amir Kaki is a paid consultant and speaker for Abiomed, Inc. He was not paid for his quote in this article.

