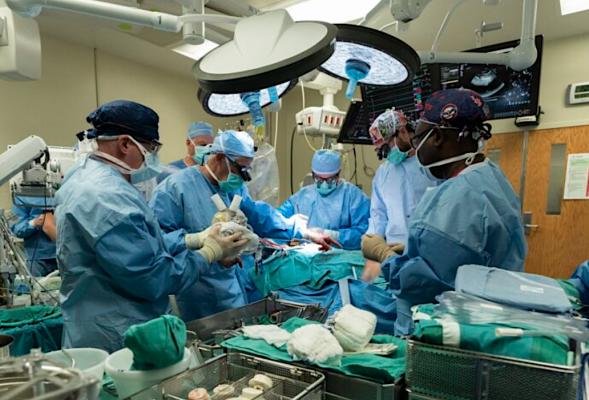
Cardiothoracic surgeons with UofL Health – Jewish Hospital and the University of Louisville performed the world’s first Carmat Aeson bioprosthetic total artificial heart implantation in a female patient on Sept. 14, 2021. The device was small enough to fit into the woman's smaller chest cavity. The device is being tested as a bridge to heart transplant rather than use of a LVAD. Photo by UofL Health.
September 22, 2021 — A cardiothoracic surgical team with University of Louisville Health – Jewish Hospital has performed the world’s first Carmat Aeson bioprosthetic total artificial heart implantation in a 57-year-old female patient. The investigational device, currently intended as a bridge to heart transplant, is part of an early feasibility study (EFS) sponsored by Carmat, a French medical device company, in partnership with UofL, UofL Health – Jewish Hospital and the UofL Health – Trager Transplant Center.
Led by cardiothoracic surgeons Mark Slaughter, M.D., and Siddharth Pahwa, M.D., both of UofL Health - UofL Physicians and the UofL School of Medicine, the team performed the implant of the device on Sept. 14, 2021, at UofL Health – Jewish Hospital. The same team completed the nation’s second implantation in a male patient last month, also at Jewish Hospital.
“For the other half of the world’s population, completion of this procedure by the Jewish Hospital team brings new hope for extended life,” said Slaughter, surgical director of heart transplant, professor and chair of the Department of Cardiovascular and Thoracic Surgery at the University of Louisville and UofL Physician at Jewish Hospital. “Size limitations can make it harder to implant artificial hearts in women, but the Aeson artificial heart is compact enough to fit inside the smaller chest cavities more frequently found in women, which gives hope to a wider variety of men and women waiting for a heart transplant and increases the chances for success.”
New Artificial Heart May Help Address Limitations of Current LVADs
More than 3,500 individuals are awaiting a heart transplant in the U.S. and 900 of them are women. There are few treatment options for patients with biventricular heart disease, meaning both the left and right sides of the heart are not pumping blood adequately.
The Aeson device is designed to solve the limitations of current left-ventricular assist devices (LVAD), which pump blood in just one chamber, by pumping blood in both heart chambers. Aeson also contains pressure sensors that estimate the patient’s blood pressure and automatically adapts cardiac output according to the sensor information. It is fully implanted as a heart replacement and powered by a portable external power supply.
During this procedure, the Aeson total artificial heart was implanted into a 57-year-old Kentucky woman with severe biventricular heart failure during an eight-hour surgery. The recipient, whose identity is being withheld upon request, was referred to the Advanced Heart Failure Therapies Program at Jewish Hospital earlier this year with end-stage heart failure and had undergone cardiac surgery years before. The patient is recovering well in the cardiovascular intensive care unit (CVICU). Jewish Hospital is just one of four programs in the nation approved to perform this clinical trial procedure.
“The varying pumping ability of the Aeson device increases its viability among more patients,” said Pahwa, UofL Physicians cardiothoracic surgeon and assistant professor in the UofL Department of Cardiovascular and Thoracic Surgery. “While other devices are set at a fixed rate or create a continuous flow, Carmat has developed the Aeson to automatically adjust the flow, creating an improved performance to meet the body’s changing blood flow needs.”
Aeson Being Tested as a Bridge to Heart Transplant
Currently, the Aeson artificial heart is tested as a bridge to transplant for patients with end-stage biventricular heart failure, allowing more time for the patient to receive a permanent heart organ transplant. The device already has been approved for such use in Europe, where approximately 20 devices have been implanted. It currently is being tested in the U.S. as part of a feasibility study approved by the U.S. Food and Drug Administration (FDA).
The first Aeson artificial heart in North America was implanted in a male patient in July at Duke University Medical Center. The second implantation, also in a male patient, was performed at Jewish Hospital in August. This third North American implantation is the first to involve a female patient.
“Even as we have fought this deadly pandemic, our researchers and health care providers have also been on the front lines of improving care and quality of life for not only Kentuckians, but for people around the world,” said Kentucky Gov. Andy Beshear. “I am proud that UofL, Jewish Hospital and their doctors are leading the world in implanting this promising and innovative device that could offer hope and time to thousands of people, including our wives, mothers and other loved ones, in coming years.”
“This third implant in the U.S. was a landmark event, not only because it allowed us to finalize the enrollment of the first cohort of patients of the EFS, but very importantly because it is the first time ever that our device has helped a woman suffering from heart failure," explained Stéphane Piat, chief executive officer of Carmat. "This achievement confirms that the size limitations for adults are minimal, which makes us very confident in Aeson’s potential to become a therapy of choice for a broad patient population.”
Preclinical research for Carmat’s artificial heart began at UofL more than five years ago. Researchers at UofL’s Cardiovascular Innovation Institute (CII) tested Aeson’s auto-regulation capability, which allows the device to adapt its flows according to the patient’s needs by detecting changes of pressure in the device. UofL researchers have conducted preclinical testing of artificial heart components and mechanical assist devices at CII for many years, testing some portion of nearly every mechanical assist device that is commercially available today.
History of Advanced Heart Failure Therapies at Jewish Hospital and the University of Louisville
Jewish Hospital and the University of Louisville have been involved in several firsts in the history in advancing heart care.
• Aug. 24, 1984: Kentucky’s first heart transplant performed at Jewish Hospital by UofL physicians
• July 2, 2001: The world’s first AbioCor artificial heart was implanted at Jewish Hospital by UofL physicians, led by cardiothoracic surgeon Laman Gray, M.D.
• Dec. 21, 2011: Kentucky’s first transcatheter aortic-valve replacement (TAVR) performed at Jewish Hospital by UofL physicians
• Jan. 18, 2015: Kentucky’s first HeartMate 3 left ventricular assist device (LVAD) implanted at Jewish Hospital by UofL physicians
• Feb. 21, 2018: UofL Health - Trager Transplant Center’s 500th heart transplant performed at Jewish Hospital
• June 14, 2019: The first EvaHeart2 LVAD implanted as bridge to transplant at UofL Health - Trager Transplant Center
• April 22, 2021: UofL Health - Trager Transplant Center’s 1,000th TAVR performed at Jewish Hospital
“This world-first artificial heart implant into a female patient is another demonstration of UofL Health’s commitment to provide both the world-class care of today and develop the world-class standards of tomorrow,” said John Walsh, chief administrative officer of Jewish Hospital. “We celebrate this first as a milestone and recognize the hard work of doctors Slaughter and Pahwa and the entire team. The true impact of their work will be measured in the dozens, hundreds and thousands of lives improved in the years to come.”
The patient who received the nation’s second Aeson implant, on Aug. 20, 2021, continues to improve at Jewish Hospital.
Related Carmat Artificial Heart Content:
Carmat Artificial Heart Shows Promising Automatic Regulation of Blood Flow
Carmat to Initiate FDA U.S. Feasibility Study of Total Artificial Heart
FDA OKs Use New Version of Carmat Artificial Heart in U.S. Early Feasibility Study
First Implantation of Carmat Total Artificial Heart in Denmark
Carmat Announces Start of the PIVOTAL Study
Find more heart failure technology news


 February 03, 2026
February 03, 2026 









