January 17, 2012 — The U.S. Food and Drug Administration (FDA) expanded the approved usage for an endovascular graft manufactured by W.L. Gore and Associates Inc. to include treatment of life-threatening tears or ruptures of the aorta (thoracic aortic transection).
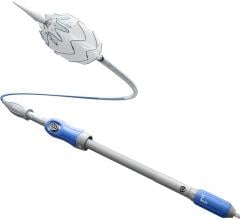
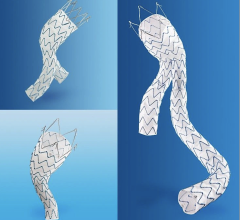
The Gore Tag thoracic endoprosthesis is the first endovascular graft approved by the FDA to treat a variety of thoracic lesions, including dangerously large bulges in the aorta (aneurysms) as well as thoracic aortic transections. The expanded approval of the Gore Tag endovascular graft provides surgeons with a minimally invasive and potentially less risky alterative to open chest surgery for the treatment of thoracic aortic transection and other lesions.
"The FDA commends the manufacturer for recognizing the need to expand the indication of Gore Tag and for proactively working to obtain the safety and effectiveness data needed to support it," said Christy Foreman, director of the Office of Device Evaluation at FDA's Center for Devices and Radiological Health.
"By obtaining FDA approval for this expanded use, physicians will now have access to risk and benefit information that can help them determine if the Gore Tag is appropriate for treating a patient with a thoracic aortic transection," said Foreman.
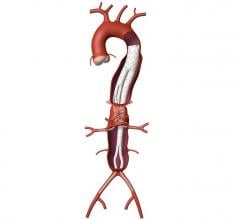
The aorta is the largest artery in the body, branching directly from the heart to supply blood to the rest of the body. A thoracic aortic transection, most often caused by a traumatic injury to the chest as a result of a motor vehicle accident, crushing of the chest, or a fall from a high height, results in profuse bleeding and is frequently fatal.
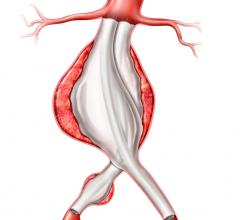
An endovascular graft is a fabric tube supported with a metal mesh frame. The endovascular graft is compressed into a long, thin, tube-like delivery catheter. The catheter is inserted into an artery in the leg and directed through the arteries to the site of the rupture or tear, where the endovascular graft is released. Once released, the endovascular graft expands against the wall of the aorta to redirect blood flow away from the tear or rupture.
FDA first approved the Gore Tag in 2005 to treat aortic aneurysms to reduce the risk of rupture. The expanded approval represents the latest design, which features materials that conform to the bends and angles common in the area where thoracic aortic transections typically occur.
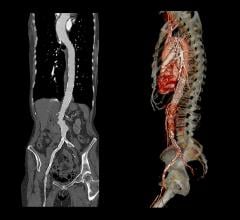
FDA based its expanded approval on data from 51 patients implanted with the Gore Tag to treat thoracic aortic transection caused by trauma. Patients were monitored for 30 days with physician exams and follow-up X-ray and computed tomography (CT) scans. All survived the implant procedure; four patients died from causes unrelated to the device or implant procedure. The company will continue to follow patients for five years.
For more information: www.goremedical.com

 April 26, 2023
April 26, 2023