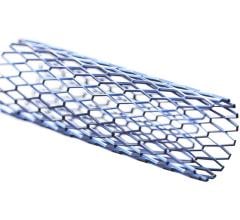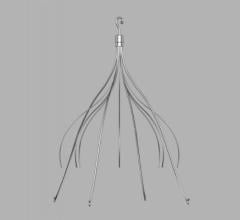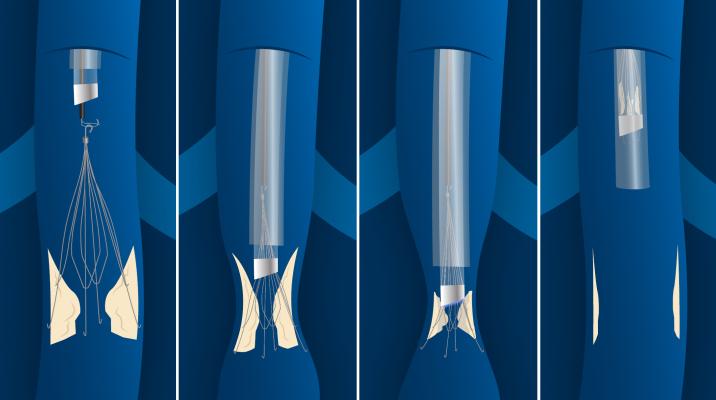
The Philips CavaClear IVC Filter Removal Laser Sheath showing how it hooks to the filter and the laser follows the IVC structure down to where it is anchored in scar tissue. It uses a CO2 laser to dissolve the issue and release the device so it can be sheathed and removed from the body.
January 4, 2022 — The U.S. Food and Drug Administration (FDA) has authorized marketing of the first laser-based device for the removal of inferior vena cava (IVC) filters. The device is designed for patients who have an IVC filter, a small cage-like device inserted into the largest vein in the body to capture blood clots and prevent them from traveling to the lungs. The Philips CavaClear Laser Sheath is intended for the removal of tissue to facilitate detachment of an IVC filter during retrieval when previous methods of removal have failed.
“To date, there have been limited options for the successful removal of chronically embedded IVC filters, as they can be difficult to retrieve due to potential complications associated with the complex procedure,” said Bram Zuckerman, M.D., director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health. “Today’s action by the FDA will provide physicians with an important tool for the safe removal of IVC filters and potentially help reduce complications for patients. It also demonstrates FDA’s commitment to leveraging real world evidence where appropriate to evaluate device safety and effectiveness.”
 IVC filters are commonly used to treat patients who are at risk for pulmonary embolism (a blood clot in the lungs) when treatment with blood thinners cannot be used or is ineffective. While some IVC filters are left in place permanently, the FDA issued a safety communication in 2014 based on reports of adverse events associated with IVC filters and recommended that implanting physicians consider removing the filter as soon as blood clots are no longer a risk for the patient.[1]
IVC filters are commonly used to treat patients who are at risk for pulmonary embolism (a blood clot in the lungs) when treatment with blood thinners cannot be used or is ineffective. While some IVC filters are left in place permanently, the FDA issued a safety communication in 2014 based on reports of adverse events associated with IVC filters and recommended that implanting physicians consider removing the filter as soon as blood clots are no longer a risk for the patient.[1]
Research has shown that IVC filters may have long-term complications.[2] The filters can fracture and travel through the bloodstream to other parts of the body. Other identified long-term risks associated with IVC filters include lower limb deep vein thrombosis and IVC occlusion.
Failure rates for IVC filter removal can be high and prior to CavaClear, limited options for removal existed if the filter became difficult to remove. Advanced retrieval tools and techniques are required if the IVC filter becomes embedded in the vasculature. Physicians previously had very few tools to remove the filter when complications occurred and until now there were no FDA-approved devices for this type of advanced removal.
“Today is a historic day. With the approval of CavaClear, physicians now have a device specifically geared remove chronically embedded IVC filters,” said Kush R. Desai M.D., FSIR, associate professor of radiology, surgery, and medicine, and director of deep venous interventions at Northwestern University Feinberg School of Medicine, Chicago. “Backed by evidence, this technology can be applied to retrieve IVC filters that are no longer indicated, reducing potential clinical risk for patients and satisfying the FDA’s guidance to retrieve filters when they are no longer indicated.”
In 2021, the FDA granted the device Breakthrough Device Designation. Laser has been clinically proven to provide a success rate over 99%, with low complication rates [3].
During removal, the Philips CavaClear Laser Sheath device is designed to facilitate detachment of firmly adherent IVC filters from the IVC wall using ultraviolet laser energy to remove a small amount of the tissue. The device is designed for use in conjunction with conventional snare devices to assist in IVC filter removal.
Clinical Research Supports Laser-assisted IVC Filter Removal
 Two independent and prospective clinical studies demonstrated that laser-assisted retrieval was 96-99.4% effective with a major adverse event rate of 0.7-2%.[3,4] Philips CavaClear uses circumferential tissue ablation that can aid in capturing the filter within seconds of laser activation, which can help increase physician efficiency during removal, and may help lower costs by reducing the number of retrieval attempts needed to remove an embedded filter. In addition, the simple and safe design is easy for physicians to integrate into their workflow.
Two independent and prospective clinical studies demonstrated that laser-assisted retrieval was 96-99.4% effective with a major adverse event rate of 0.7-2%.[3,4] Philips CavaClear uses circumferential tissue ablation that can aid in capturing the filter within seconds of laser activation, which can help increase physician efficiency during removal, and may help lower costs by reducing the number of retrieval attempts needed to remove an embedded filter. In addition, the simple and safe design is easy for physicians to integrate into their workflow.
The FDA assessed the safety and effectiveness of the device through a retrospective, real-world evidence clinical study. The study, which evaluated laser-assisted IVC filter removal in 265 patients at seven clinical sites, demonstrated a procedural technical success rate of 96%. The study had a 3% rate of significant device-related complications, including IVC injury causing extravasation (bleeding), hematoma formation (bleeding outside of the blood vessel), and filter breakage.
Use of the CavaClear Laser Sheath device is contraindicated when a blood clot is present within the filter or surrounding veins, when the IVC filter is not accessible, or when the filter is nonmetal. The device may not be used in removal of Bird’s Nest IVC filters and VenaTech IVC filters.
The FDA reviewed the device through the de novo premarket review pathway, a regulatory pathway for some low- to moderate-risk devices that are novel and for which there is no legally marketed predicate device to which the device can claim substantial equivalence.
References:


 November 13, 2025
November 13, 2025