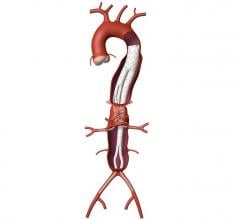
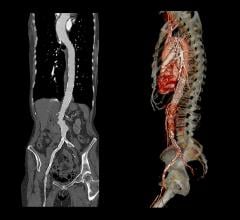
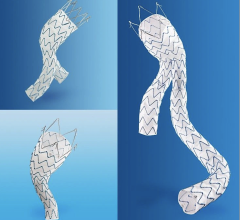
November 24, 2008 - Endologix Inc. today said results from its Powerlink XL system for the minimally invasive treatment of abdominal aortic aneurysms (AAA) prospective, multi-center clinical trial met the primary endpoint of one-year post-procedure freedom from Type I proximal endoleak.
Additionally, no stent graft distal migration was observed in any study patient at one year following the procedure, the company said. The trial was conducted under an investigational device exemption (IDE) approved by the FDA and the Powerlink XL is currently being commercially launched in the U.S.
Additional one-year follow-up results for the Powerlink XL study include zero aneurysm-related mortality, aneurysm rupture, or conversion to open repair. Independent Core Lab evaluation reported no ePTFE graft disruptions, stent fractures, or Type III or IV endoleaks. The study also showed stable or significantly reduced aneurysm sac diameter in 96 percent of patients.
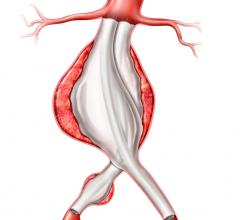
Data from the Powerlink XL trial were presented today at the 35th Annual VEITHsymposium by the trial’s principal investigator professor William D. Jordan, Jr., M.D. , chief of vascular surgery, UAB Hospital, Birmingham, AL. Dr. Jordan reviewed results of this approach to endovascular treatment of patients with aortic necks up to 32mm in diameter. All patients received the Powerlink bifurcated device via anatomical fixation at the aortic bifurcation, as well as the Powerlink XL proximal extension to achieve proximal seal.
“These impressive data are consistent with the positive results reported by other clinicians who have used the Powerlink System to treat patients with aortic necks up to 26mm,” Dr. Jordan said. “Importantly, for the first time, we were able to demonstrate in a designed study the benefit of an AAA endograft in achieving secure fixation at the bifurcation with proximal seal in a patient group with large aortic necks.”
He said the results are significant given the relatively high prevalence of hostile neck anatomy with severe thrombus and/or a reverse taper, which occurred in more than 80 percent of patients.
For more information: www.endologix.com

 April 26, 2023
April 26, 2023