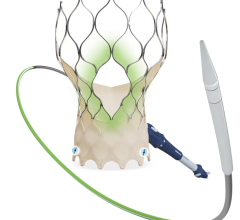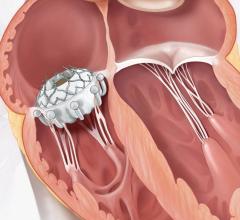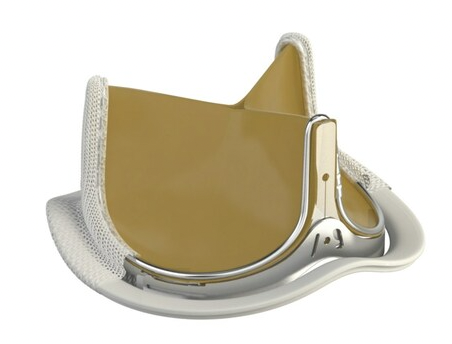
INSPIRIS RESILIA aortic valve
May 9, 2023 — Edwards Lifesciences announced new data from the COMMENCE aortic trial, demonstrating low rates of structural valve deterioration (SVD) in bioprosthetic aortic valves with the company's innovative RESILIA tissue. The data, which represent the longest clinical follow-up for Edwards' bioprosthetic surgical aortic valve with RESILIA tissue with a mean follow-up of 7.7 years, were presented today at the 103rd annual meeting of the American Association for Thoracic Surgery.
SVD can be caused by a buildup of calcium that may impact long-term durability of bioprosthetic valves. Heart valves with RESILIA tissue are designed to address calcification challenges of conventional tissue valves, potentially allowing valves with RESILIA to last longer. The RESILIA tissue data from the COMMENCE aortic trial reported encouraging results with low rates of SVD (99.3% freedom from SVD), clinically stable gradients and freedom from reoperation (97.2%) through seven years.
"As bioprosthetic aortic valve replacement extends to younger and more active patients, valve durability is becoming increasingly important," said Thomas Beaver, M.D., Grant and Shirle Herron Chair, Professor and Chief of Cardiovascular Surgery, University of Florida College of Medicine. "The seven-year data from the COMMENCE aortic trial demonstrates strong clinical outcomes and excellent durability in a study of younger patients with a mean age of 65.1 years."
Current technologies utilizing this novel tissue include the INSPIRIS RESILIA aortic valve, the KONECT RESILIA aortic valved conduit, the MITRIS RESILIA mitral valve and the SAPIEN 3 Ultra RESILIA transcatheter aortic heart valve. In addition to its anti-calcification properties, RESILIA tissue also allows the valve to be stored under dry packaging conditions, facilitating ease of use in the operating room.
"Edwards Lifesciences is committed to addressing the needs of patients with structural heart disease and our RESILIA tissue technology is designed for enhanced durability, supporting patients' improved quality of life," said Larry Wood, Edwards' corporate vice president and group president, transcatheter aortic valve replacement and surgical structural heart. "The latest COMMENCE aortic trial data emphasizes the value of RESILIA tissue-based offerings in helping patients and their physicians with lifetime management of valve disease."
The latest data from the COMMENCE aortic trial join a growing library of clinical studies in support of RESILIA tissue.
The COMMENCE aortic trial is a prospective, non-randomized, multicenter, single-arm investigational device exemption (IDE) trial comprised of 689 patients at 27 clinical sites across the United States and Europe. The trial is evaluating the safety and effectiveness of Edwards' bioprosthetic aortic valve with RESILIA tissue in patients ages 18 and older with diagnosed aortic valve disease and scheduled to undergo aortic valve replacement surgery. Long-term follow-up data were collected in a subset of these patients and will continue to be evaluated through 10 years.
For more information: www.edwards.com


 July 08, 2024
July 08, 2024