September 29, 2010 - Preclinical data of a therapy designed to treat drug-resistant hypertension was presented last week at the Transcatheter Cardiovascular Therapeutics (TCT) 2010 symposium in Washington, D.C.
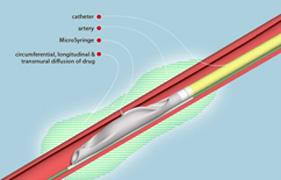
The therapy, by Mercator MedSystems, combines the use of the company's Bullfrog Micro-Infusion Catheter with a drug to reduce the hyperactivity of nerves in the renal artery leading to and from the kidney. These nerves are implicated in the initiation and maintenance of high blood pressure. Early data show that this therapy precisely targets these nerves, achieving the desired effect without negatively affecting surrounding tissue.
"While these data are early, the site-specific delivery of drugs directly to the nerves in question offers great promise for a new approach in treating the nearly one-third of 73 million U.S. hypertensive patients who cannot control their blood pressure with medication alone," said Christopher D. Owens, M.D., MSc, assistant professor in residence, division of vascular and endovascular surgery, University of California, San Francisco. Owens presented the data from the study.
During the procedure, the micro-infusion catheter is introduced into an artery through a small needle in the upper leg and guided into the renal artery in the kidney. Once positioned, a balloon at the tip of the catheter is inflated inside the artery, sliding a 130-micron (two hair widths thick) microneedle through the artery wall into the tissue known as the adventitia, which envelops the hyperactive nerves leading to the kidney. As the drug is infused through the microneedle, it surrounds the artery to create a cylindrical treatment zone and reduces the nerve signals that cause high blood pressure.
Human trials are planned for early 2011.
For more information: www.mercatormed.com


 January 05, 2026
January 05, 2026 









